Fluorescence microscopy plays an important role in biological studies, allowing scientists to capture detail with high cellular imaging resolution and labeling specificity. However, in its simplest embodiment (epifluorescence microscopy), this technique lacks the ability to optically section samples for true 3D imaging, as it receives both in-focus and out-of-focus signals. To acquire 3D image datasets with optical sectioning, light sheet fluorescence microscopy (LSFM) and laser scanning confocal microscopy (LSCM) are commonly employed. These imaging methods vary in how they acquire fluorescent signals throughout the samples, leading to significant differences in imaging speed and the resulting data quality.
What is laser scanning confocal microscopy?
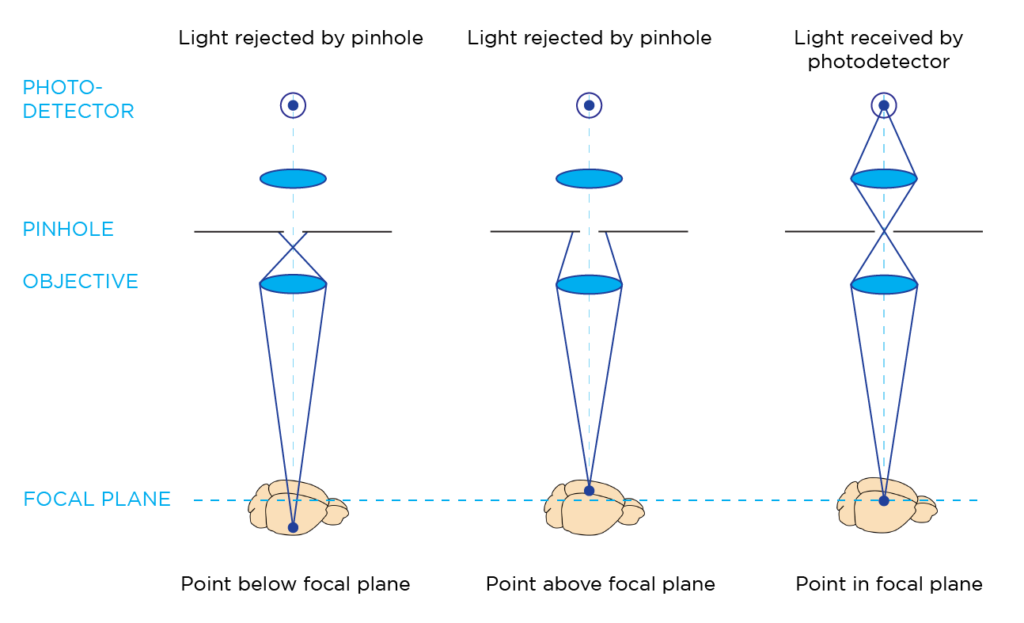
Laser scanning confocal microscopy enables the visualization of biological specimens by using focused illumination and detection optics that converge onto a diffraction-limited spot in the sample. This approach typically employs a single objective lens that serves for both illumination and detection in a confocal setup. A confocal pinhole selectively blocks out-of-focus light, ensuring that the photodetector (usually a photomultiplier tube) only captures fluorescent signals from the focal point, resulting in optical sectioning for 3D imaging.
How do laser scanning confocal microscopes acquire images?
A conventional laser scanning confocal microscope relies on galvanometric mirrors to raster-scan the illumination beam across the field of view (FOV). This process illuminates each pixel individually in sequence, with the photodetector collecting light from each pixel to assemble the final image.
What are the overall advantages of laser scanning confocal microscopy?
Laser scanning confocal microscopy is a powerful imaging technique that is particularly well-suited for capturing high-resolution images of smaller samples. This technique is compatible with high-NA objectives, which are highly effective at capturing emitted light at the focal plane, resulting in high image resolution.
What are the limitations of laser scanning confocal microscopy?
Laser scanning confocal microscopy can offer high-resolution imaging for many applications, but slow imaging speed and the potential for photobleaching can limit feasibility in larger samples. LSCM typically acquires images in a point-scan manner, which can take several seconds for an entire FOV. When imaging whole cleared organs in 3D on the millimeter or centimeter scale, this approach becomes impractical as the imaging time scales up to weeks or even months.
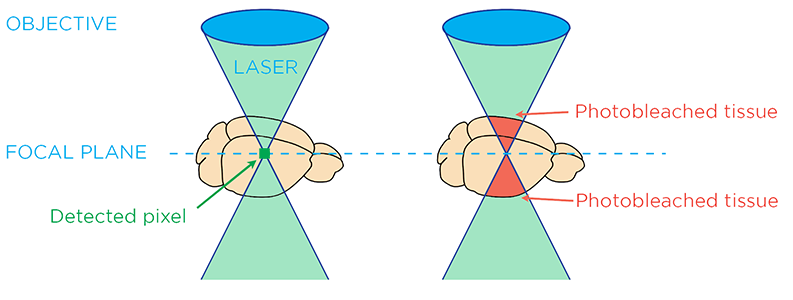
While increasing the illumination intensity can decrease exposure time and increase the imaging speed, it also leads to a process called photobleaching, which quickly degrades the fluorescent probe signal. Although the pinhole in laser scanning confocal microscopy effectively blocks out-of-focus light from detection to achieve optical sectioning, tissue above and below the focal plane still receives a significant amount of illumination light. This can lead to photobleaching of the sample outside the focal plane, compromising the imaging quality of cleared organs.
What is light sheet fluorescence microscopy?
Light sheet fluorescence microscopy employs planar illumination to illuminate the entire FOV at once. By effectively decoupling the paths of illumination and detection with separate light paths, only one z plane of the sample is illuminated at a time. This allows for optical sectioning and volumetric imaging with reduced photobleaching and low to zero background from other z planes.
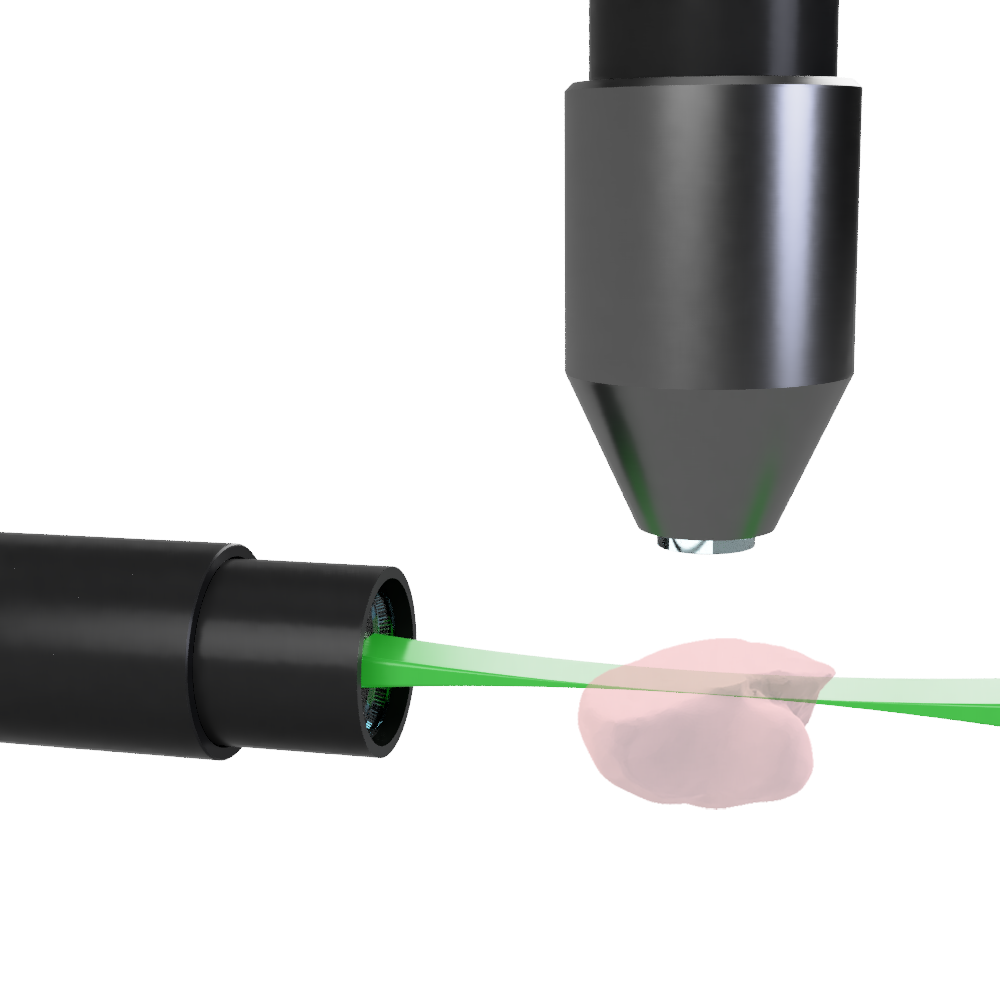
How do light sheet fluorescence microscopes acquire images?
A typical light sheet fluorescence microscope has illumination and detection objectives placed orthogonally. The centers of each focal plane are coincident such that the illumination sheet of light is focused under the detection objective at its focal plane in order to illuminate the z plane at the FOV.
"[LSFM] allows for optical sectioning and volumetric imaging with reduced photobleaching and low to zero background from other z planes."
The traditional approach to creating a light sheet is referred to as the static light sheet method. This method utilizes a cylindrical lens to focus the illumination light in one dimension and create a planar illumination.
This technique enables the camera to capture a fully illuminated plane in a single exposure, allowing for high-speed scanning of the sample through the illumination beam. As a result, static light sheet generation achieves exceptionally high frame rates for volumetric imaging.
Overall, how does light sheet fluorescence microscopy compare with laser scanning confocal microscopy?
Light sheet fluorescence microscopy is particularly effective for high-throughput volumetric imaging, with lower potential for photobleaching. Laser scanning confocal microscopy is typically two to three orders of magnitude slower than LSFM, and photobleaching can result from illuminating the entire tissue thickness to acquire only one section of the z-plane.
The SmartSPIM and MegaSPIM light sheet microscopes are uniquely optimized for cleared tissue imaging. With patented axial sweeping technology, both SmartSPIM and MegaSPIM generate uniform axial resolution across the entire FOV, offering industry-leading image quality and acquisition speed for a wide range of research applications. With our systems, you can easily capture high-quality images of a mouse brain hemisphere or similarly sized intact sample in just 30 minutes. Reach out to an imaging specialist today to learn more!