Dr. Michelle Bedenbaugh is a Postdoctoral Research Fellow in Dr. Richard Simerly’s lab at Vanderbilt University.
LifeCanvas (LC): What is your current research focus and how did you become interested in it?

Michelle Bedenbaugh (MB): Before joining the Simerly lab almost 5 years ago, I worked on reproductive circuits and the neuroscience of female puberty. I wanted to learn more about the shared neural circuitry of reproduction and other homeostatic mechanisms, specifically feeding. When I joined the lab, Dr. Simerly was collaborating with Dr. Roger Cone at the University of Michigan, looking at the potential role of the melanocortin-3 receptor (MC3R) at the nexus of communicating metabolic state and reproduction.
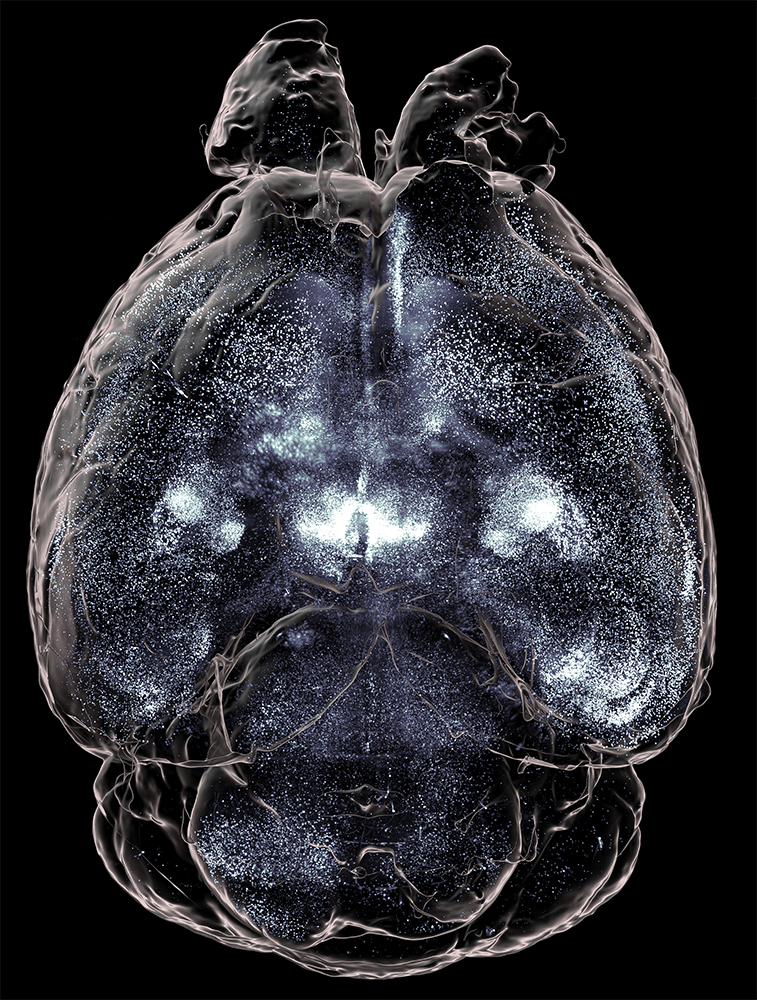
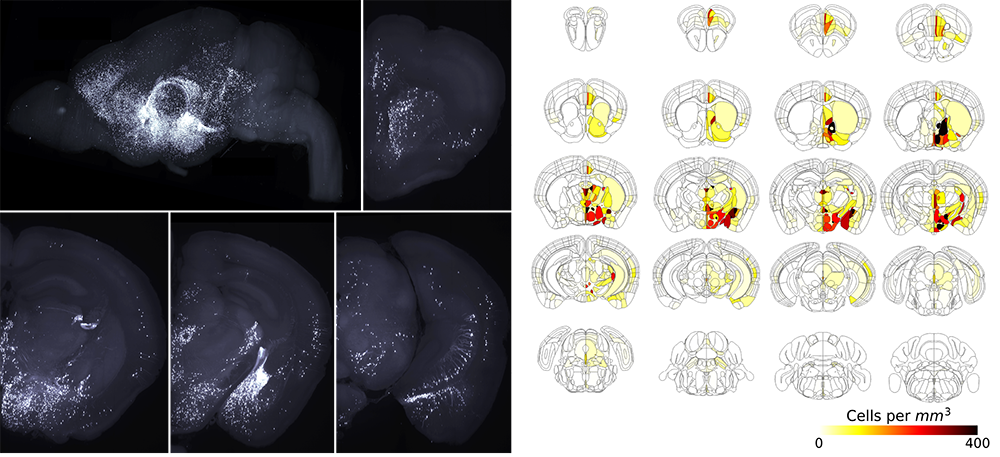
To begin understanding this in a brain-wide fashion, we cleared and imaged whole brains with light sheet microscopy. We found that MC3R neurons were present in brain regions heavily involved in stress and anxiety circuits, as well as feeding and reproduction (Bedenbaugh et al. 2022).
Now, much of my work investigates the involvement of MC3R in stress, feeding, and anxiety circuits. I focus on a region called the bed nucleus of the stria terminalis (BNST), a hub for behaviors related to these circuits. I’m looking to map MC3R circuits from the BNST to other areas of the brain, in order to understand their role at the intersection of feeding and anxiety-related behaviors.
LC: What kinds of behavioral changes do you observe when you manipulate these MC3R neurons?
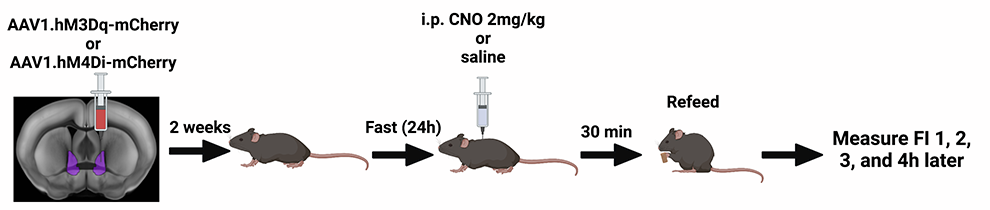
MB: We are using a tool called DREADDs (Designer Receptors Activated Only by Designer Drugs) to pharmacologically excite or inhibit MC3R neurons in the BNST and look at behavioral effects. We have found that if we excite MC3R neurons in the BNST of mice that have fasted for 24 hours, they eat less at their next feeding than mice which have not been chemogenetically stimulated. This appears to be more pronounced in males than females. We also see that MC3R-excited mice struggle less in the restraint stress paradigm than their control counterparts.
In an earlier study collaborating with the Cone lab (Sweeney et al. 2021), we completely knocked out MC3R in both male and female mice and found, particularly in females, a notable increase in susceptibility to anxiety-type phenotypes. This is interesting because, when we hone in on manipulating neurons in the BNST, we see changes mainly in feeding and stress behaviors, and not so much anxiety. This may mean that there are other neural circuits driving anxious behavior, while this pathway is more specific to feeding and stress.
LC: How has whole brain mapping with LifeCanvas’ tools helped you develop and pursue your research questions?
MB: Because MC3R was a relatively uncharacterized neural system when our work began, a whole-brain approach was necessary. Initially, SmartSPIM light sheet microscopy functioned as a discovery tool, allowing us to obtain expression data across different brain regions very quickly. Now, we are working on using light sheet data in a quantitative manner, looking at brain-wide expression differences as well as network wiring differences between males and females.

High-quality analysis depends on good registration (with the Allen Brain Atlas) and signal-to-noise ratio, which in turn depends on high-quality samples and images. SmartSPIM and SmartAnalytics enable all of these components. Without the ability to readily examine intact 3D structures, we would lose substantial data and time. Even though I’ve looked at up to 100 MC3R brains by now, I can still find something new.
LC: Are you interested in collaborating with clinical researchers in the future?
MB: Compounds identified in the Sweeney et al. paper have actually led to the development of an MC3R agonist, which is now in clinical trials for the treatment of anorexia. We would like to use these potent MC3R agonists and antagonists to look at brain-wide cell activation, which would definitely have translational relevance. Collaborating with clinical researchers and/or drug companies could be a great opportunity to learn more about where these compounds are acting, what cells they affect, and their therapeutic potential.
In my opinion, using a whole-brain perspective is key to designing translationally relevant studies. The brain is incredibly complex and interconnected, and while this makes it difficult to simplify for research purposes, this complexity is foundational to how brains work.
LC: What are your next steps?
MB: I plan to use monosynaptic rabies tracing (MRT), as described in our collaboration with Dr. Danny Winder’s lab (Luchsinger et al. 2021), to specifically look at neurons that convey information to MC3R neurons in the BNST. I want to quantitatively compare whole networks between males and females, as well as behavioral effects in feeding, stress, and anxiety behaviors and physiology.
"... Using a whole-brain perspective is key to designing translationally relevant studies ... Complexity is foundational to how brains work."
Further in the future, I also want to examine how developmental perturbations in early life, such as changes in diet or stress, affect neural circuits across the whole brain in males and females. This could help us understand how certain experiences during critical developmental periods affect brain circuitry and behavior.
LC: What are some of your key takeaways from SfN this year?
MB: I’m always struck by the new tools that appear at SfN, which was amplified this year by the fact that we haven’t seen each other in person since 2019. There’s been quite a lot of development in optogenetics, as well as techniques for live behaving animals such as micro-endoscopy and fiber photometry. It’s exciting that we’re starting to be able to look at multiple areas at once, and how whole circuits are interacting to generate complex behaviors. Before joining the Simerly lab, I actually worked with large animals like sheep and cattle – I’m always amazed at how sophisticated the technology is for working with rodents.