LifeCanvas (LC): Could you describe the academic journey that led to your current research?
Sam Centanni (SC): As an undergraduate, I was working on understanding salivary gland morphogenesis using confocal microscopy. Seeing how the glands branch as they develop, and how different compounds affected this development, got me initially interested in fluorescent imaging techniques.

In graduate school, I pivoted to addiction neuroscience, studying the lasting effects of adolescent alcohol use on adult cognition and behavior. I continued to use visual approaches like confocal microscopy and dendritic spine morphology to look at neuroadaptations that can occur after alcohol use. In my postdoc, I worked on the neurocircuitry regulating some of these alcohol-induced effects – specifically, what happens during drinking that can persist into abstinent periods.
Now, I’m interested in the interaction between stress and alcohol, which have many overlapping effects on neural circuits. What happens to these neural circuits before alcohol exposure, and how can this potentially predict a predisposition to alcohol use disorder?
LC: How do you assess the effects of alcohol use on mice?
SC: Our research focuses on voluntary models of alcohol use: giving mice a choice between alcohol and water in their home cages and looking at passive consumption. We then look at differences in neural circuits between mice that choose water and mice that choose alcohol, as well as neuroadaptations that occur in response to voluntary drinking.
"... It’s been exciting to watch this pipeline develop to become more streamlined and comprehensive – it’s truly end-to-end, from clearing and imaging through analysis."
We’re most interested in what happens to emotional or affective states after the alcohol is gone. Abstinence in this context can be a period of hypersensitivity to stress, where even a seemingly innocuous stressor can trigger relapse. We don’t really understand the mechanisms of this sensitivity and relapse response. With our models and tools, we can manipulate circuits to determine what’s driving this hyperactive stress response system.
LC: What led you to adopt light sheet imaging techniques in your research?
SC: As a postdoc in Danny Winder’s lab, we were lab neighbors with Richard Simerly, whose lab was using light sheet microscopy for whole-brain imaging. Along with my colleague Joe Luchsinger, I saw that light sheet imaging could be a powerful tool for discovering upstream inputs regulating our areas of interest. This unbiased approach was much more appealing to me compared to parsing the literature for all the different upstream targets for each region, formulating a hypothesis, and then using tissue sectioning and confocal microscopy to assess that hypothesis. Since then, we’ve been using light sheet techniques in our lab in multiple ways.
I also think light sheet microscopy can be a very effective teaching tool. I’m personally a visual learner, and I find it much easier to visualize these pathways in 3D-rendered images versus looking at projections in old papers. You can recapitulate decades of data in 3D in a fraction of the time, and you don’t have to be a neuroanatomist to understand it.
LC: What tools are you using to map these neural circuits, and what have you learned so far?
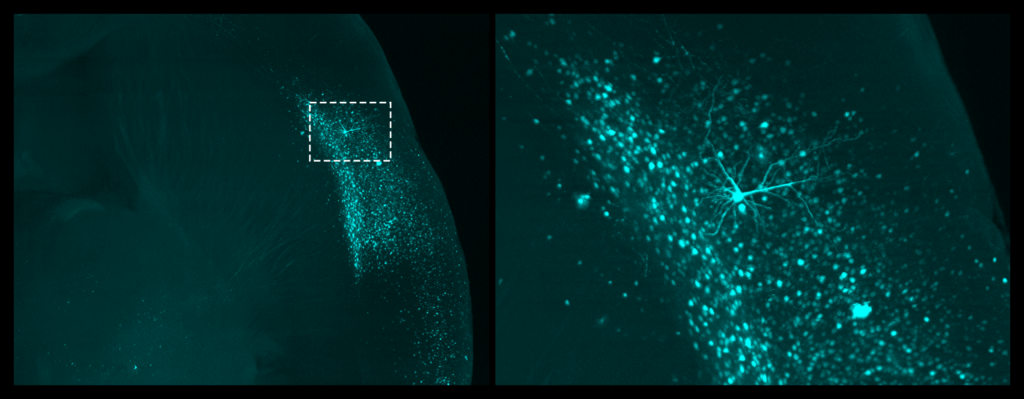
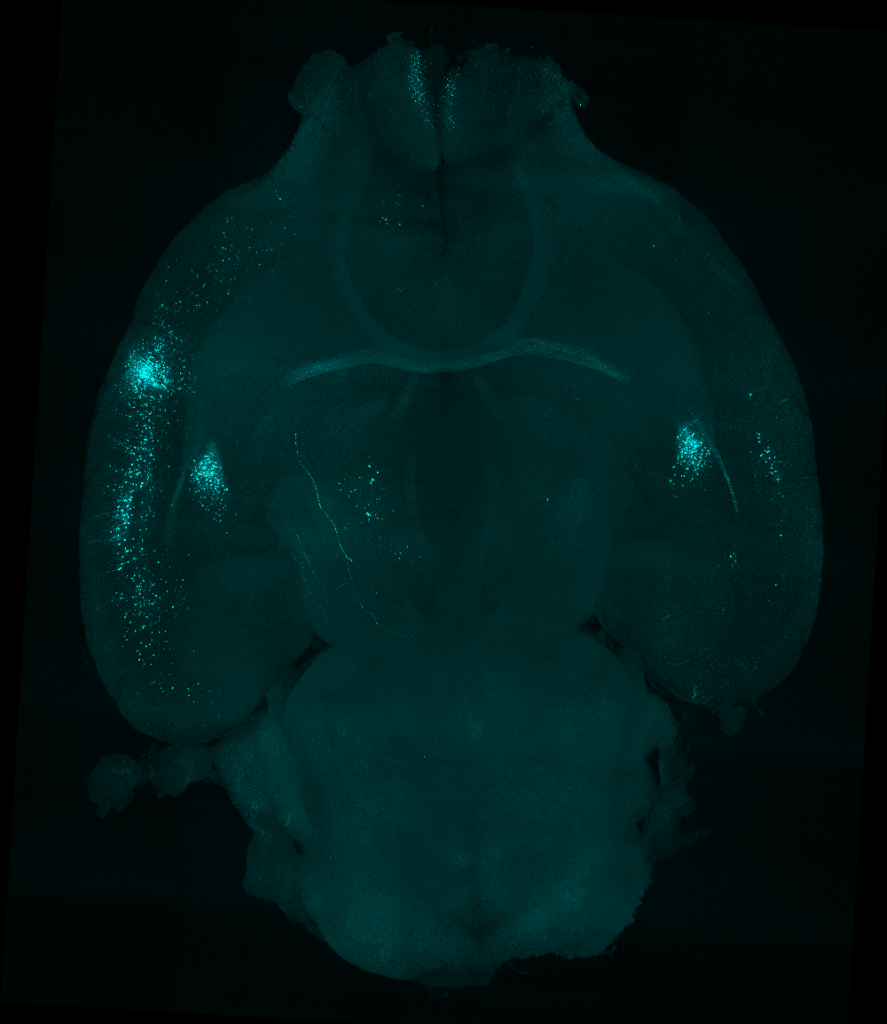
SC: We used viral approaches to map circuits in a brain region called the bed nucleus stria terminalis (BNST), which is known to be involved in emotional regulation and thus could be driving emotional negative affect during abstinence. Wanting to understand what upstream inputs were regulating this effect, we injected a retrograde tracer into the BNST, and used light sheet microscopy to explore the projections. We identified an insular cortex projection, which hadn’t really been studied in the context of alcohol use disorder, and showed that it drove hyperactivity in the BNST during abstinence.
After this study, we moved upstream and looked at third order inputs: projections onto the insular cells that project onto the BNST! This new world of circuit-specific inputs gave us many different candidates that could be regulating this pathway.
In a 2021 paper, we identified a motor cortex control network that inputs onto this insular pathway, controlling stress response and active coping behavior. In the future, we are also interested in looking at sensory and thalamic inputs, which we would not have been able to identify without light sheet imaging.
LC: Are you delving into cell-level contributions as well?
SC: We’re advancing our viral strategies with a genetic mouse line that uses a technology called TRAP (Targeted Recombination in Activate Population) to isolate specific ensembles of cells active during a temporally restricted window. This mouse line allows you to “trap” individual cells during a behavior by expressing a Cre-recombinase when c-FOS expression is detected. We can then express a Cre-dependent fluorescent tag, like tdTomato, to label infected cells activated during that behavior. Currently, we are using this technique to look at stress ensembles in the insula. Once these cells are tagged, we can use other tools to determine their unique physiological and molecular properties, and how manipulating them can impact other behaviors like alcohol drinking.
Combining this technology with light sheet imaging is another major focus of the lab. We can infect these stress-activated cells with retrograde viral tracers and map upstream brain areas that project specifically onto them. I’m excited to compare general projections from our discovery-oriented studies with the specific behavioral circuits or ensembles from these newer studies. I think neuroscience should be less focused on the boundaries between brain regions, and more on the interactions between these nested, interdependent circuits. We now have the ability to look at the effect of a handful of cells on the entire brain and, consequently, behavior.
LC: Your current focus is on alcohol use disorder, but how could your research help us understand stress more broadly?
SC: Studying the parallels between stress response, drinking, and the development of negative affect during abstinence can help us better characterize predisposition to alcohol misuse, and consequently stress susceptibility. Looking closely at the neural circuits involved in these behaviors can also help us dissect individual differences in stress responses. We all experience some level of stress every day – it’s important to understand how this can lead certain people to develop stress-related disorders like alcohol abuse, PTSD, and so on. Clinical researchers can benefit from our approach by using fMRI or TMS to identify and target regions or circuits activated by a specific stressor in an individual. So there are opportunities for translational studies as well.
LC: How has the LifeCanvas pipeline helped streamline your lab workflow and push your studies forward?
SC: I’ve been working with LifeCanvas for about 5 years, between Vanderbilt and my own lab at Wake Forest, and it’s been exciting to watch this pipeline develop to become more streamlined and comprehensive – it’s truly end-to-end, from clearing and imaging through analysis. The LifeCanvas team is incredibly quick and receptive. If we have a problem, they’re on a call with us in a minute. If an analysis model isn’t working well, they’re right there to figure out why, and to make the necessary tweaks – even if the changes are unique to our research interests.
As a postdoc, I wanted to do everything myself. Now that I run my own lab, I’ve realized the value of having an external resource for training and support, and being able to use my time in other ways. The LifeCanvas team has also been a constant from my postdoc into my current lab, which made the transition a lot easier. One of the first major pieces of equipment I set up was SmartSPIM.
"... Light sheet microscopy can be a very effective teaching tool ... You can recapitulate decades of data in 3D in a fraction of the time, and you don’t have to be a neuroanatomist to understand it."
LC: What are your next steps?
SC: I would like to be able to quantify whole-brain changes in neural activity using light sheet microscopy. I’m excited to pursue whole-brain analysis of c-FOS expression – with both TRAP and immunolabeling methods – as well as co-localization with specific circuits and cell ensembles.
We are also working on genetic approaches that can isolate individual neurons specific to certain projections. What’s unique about these circuits, and how are they impacted by stress and alcohol use at a transcriptomic level? Can we identify targets within these cells that may be useful for mitigating some of these effects?
Additionally, we are excited to look at novel circuits. Light sheet imaging allows us to continue identifying new targets in the context of our models, and look upstream at potential regulatory mechanisms for these stress and reward-related circuits. There isn’t much work on the impact of nontraditional areas, like sensory inputs, on these kinds of behaviors.