Active Delipidation with SmartClear II Pro
Reagents Required
- SmartClear II Pro
- Delipidation Buffer – stored at RT
- Conduction Buffer – stored at RT
- SmartClear Membranes – v2 Required
Protocol
Day 0
1. Incubate the samples in Delipidation Buffer for 24 hours at 45°C. If a 45°C shaker is not available, use 37°C.
[Note] Only 400 mL of solution will be used in the device, so 100 mL can be removed from the bottle to be used for this purpose. This 100 mL can be re-used multiple times.
Day 1
2. Prepare the device.
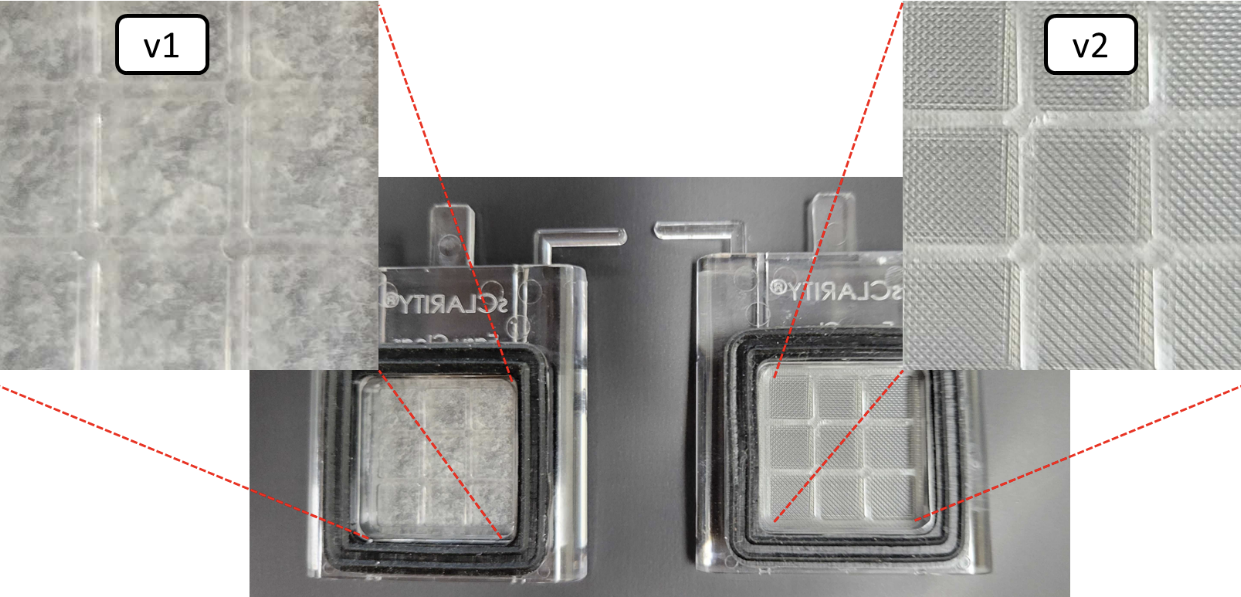
- This protocol requires v2 membranes. This will be specified on the bag containing the membranes. Please confirm the membranes are the correct version before proceeding!
- [Note] Use of older membrane types will result in rapid pH drop and reddening of samples. The v2 membranes also have a grid pattern on the membrane (see image below).
- Install membranes and buffer in the SmartClear II Pro. In short, install membranes and spacers and pour Delipidation Buffer into Reservoir A, Conduction Buffer into Reservoir B after testing the membranes with distilled water. See Buffer and Membrane Installation for details.
4. Prepare the samples.
- Insert into an appropriately sized mesh bag.
- It is best to align the sample with the longest dimension vertically for best clearing speed (cerebellum down is best). Use the notches at the top of the mesh bags to identify samples.
5. Load the samples into the device.
- Insert the mesh bag into the cylindrical sample holder and insert that in the clearing chamber. It is best to use the dividers to split the holder into quarters.
6. Prepare the device for clearing.
- Tighten the knob on the clearing chamber and close the lid of the device.
- Set the temperature accordingly.
- If your samples contain endogenous fluorescence: clear at 42°C for Buffer A (Delipidation Buffer).
- This is equivalent to the ‘Gentle’ setting in beginner mode.
- If your sample doesn’t have endogenous signal, you can increase the temperature of Buffer A to 50°C, or ‘Fast’ setting for faster clearing speed.
- [Note] If you want further control of temperature, operate the device in Expert Mode. For preservation of RNA for future FISH studies, clearing should be performed at 37°C. To operate at this temperature, you may need to reduce the current from 1500 mA to 1000 or 1200 mA.
- If your samples contain endogenous fluorescence: clear at 42°C for Buffer A (Delipidation Buffer).
- Turn on Electrophoresis power and clear the samples. The samples will not appear transparent at this stage, and they will almost appear unchanged.
- Set the clearing time. This is dependent on the sample.
- Adult mouse brains will clear in 30 hours at 42°C.
- Rat brains will clear in roughly 7 days at 42°C.
- Smaller samples will clear faster.
[Note]: Unique Tissue Types
- If your sample is liver, we have found that an additional step will result in more transparent samples. Please follow the Liver Clearing Protocol to complete clearing of livers after Step 1 in the protocol above.
- If your sample contains significant connective tissue, adipose tissue, or skin, (such as whole mouse heads) we have found that an additional dehydrating clearing step helps clear these other tissue types without damaging fluorescent proteins. Please follow the fDisco protocol after completing Step 1 in the protocol above. Please note that this step has the potential to harm antigenicity. This step can also be moved to after immunolabeling if you find that to be the case.
7. Transfer to PBS with 0.02% sodium azide at the end of the experiment.
- Store the samples at 4ºC until you are ready for the next steps.
8. Wash and shut down the device.
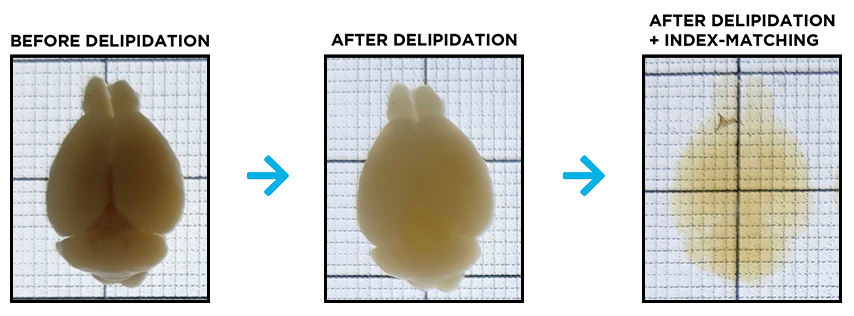
At the end of delipidation, the samples will look the same as before clearing and should not have changed size. You will not be able to tell that the sample is done clearing until the index matching stage. See images below.
You have finished Delipidation and may now move on to the next step. If your sample requires labeling, move on to that section. If no labeling is required, you can move on directly to Index Matching.
