LifeCanvas (LC): What is your current research focus?
Rafael Perez (RP): I study the effects of environmental cues on opioid tolerance. As people (and mice) consume opioids over time, they build tolerance. If they are used to receiving opioids in a particular context, they will actually lose some of that tolerance when they take the drug in a different context. This can have detrimental and even fatal effects; for example, high opioid tolerance might protect someone against the drug’s respiratory impact in a familiar context, but as their tolerance drops in a new or unassociated context, they are more susceptible to respiratory failure.

There have been decades of behavioral research confirming this phenomenon, however the neurobiological mechanisms underlying the environmental modulation of opioid tolerance are practically unknown. My work has focused on understanding these processes and how they can be modified to adjust tolerance.
LC: How did you become interested in opioid tolerance?
RP: I have always been interested in addiction and psychiatry. As an undergraduate, I studied the effects of chronic social defeat stress on epigenetic regulation of neurons and became fascinated by the idea that non-physical factors can dramatically alter the brain. At Vanderbilt, I worked with Dr. Danny Winder to look at how stress can precipitate addiction relapse. Specifically, we looked at how guanfacine, an inexpensive and widely available ADHD medication, could prevent stress-induced relapse of cocaine seeking. This research involved modeling the experience of individuals that are already far along in the addiction process, however my current work examines much earlier stages of addiction.
LC: How do you behaviorally assess a mouse’s tolerance and the impact of environmental context?
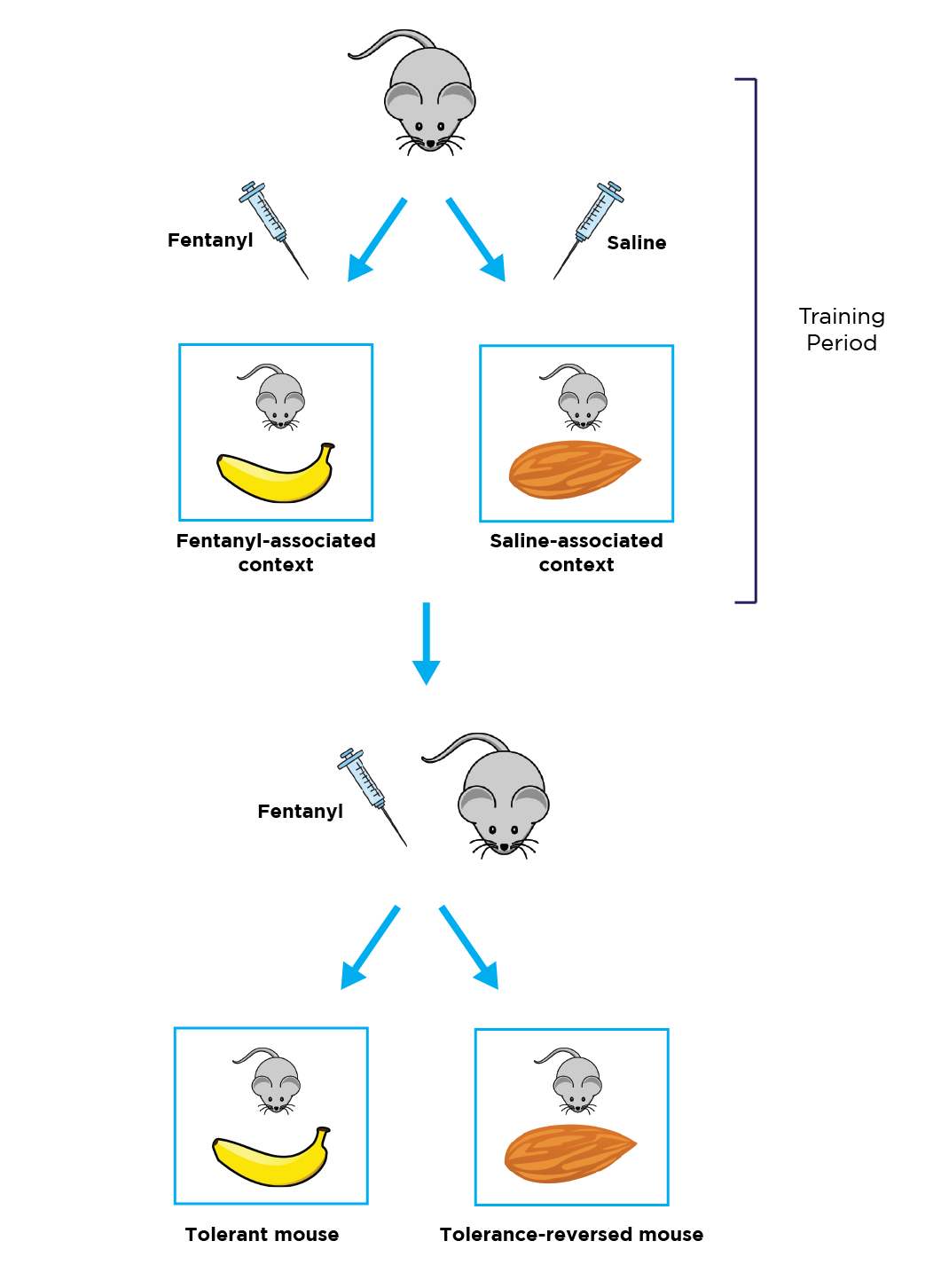
RP: In our paradigm, we inject mice with a very low dose of fentanyl to induce some level of analgesia, or saline as a control, and then place them in a room with a hot plate and specific environmental cues, such as texture and smell. For example, one room smells like bananas and has a soft floor, while another room smells like almonds and has a hard floor. Pilot experiments confirmed that these cues on their own don’t alter baseline opioid response or pain sensitivity. To create an environmental association between the banana room and the fentanyl, we repeatedly inject mice with fentanyl only in the banana room, and we inject them with saline only in the almond room.
We use the hot plate assay to assess thermal pain sensitivity in the mice throughout the experiment, which acts as a proxy for opioid tolerance. The first day, fentanyl-injected mice have a 95-100% response to the opioid, which is indicated by a long delay in hot plate reaction time. But over several sessions across two weeks, we see a dramatic decrease in opioid response. By day 14, there is a 50% reduction in response delay. In between the fentanyl sessions, we are injecting the mice with saline in the almond room, priming them to anticipate more pain in that environment.
Even a month after this training period, we can place the mice in the banana room and still see a 50% response to the fentanyl, indicating a consistent, long-lasting opioid tolerance. However, if we give mice the same dose of fentanyl in the almond room where they are used to receiving saline, we see a remarkable reversal of tolerance: mice exhibit a 75-80% response to the opioid again.
"LifeCanvas brain mapping has been instrumental in helping us cast the widest net possible in order to discover what brain regions are involved in building tolerance, and consequently identify potential targets for manipulation."
Furthermore, we see the same reversal of tolerance even in an entirely new context (e.g. a room that smells like oranges). Opioid tolerance only occurs in the room which has been associated with chronic fentanyl injections, indicating a context-dependent effect.
LC: What kinds of differences do you see in the molecular phenotypes of these mice, and how has whole brain mapping helped you identify them?
RP: Compared to more thoroughly-studied behaviors like fear conditioning and social defeat, there is substantially less literature on the brain regions involved in opioid tolerance. LifeCanvas brain mapping has been instrumental in helping us cast the widest net possible in order to discover what brain regions are involved in building tolerance, and consequently identify potential targets for manipulation.
Based on the c-FOS panel and literature, we found a few brain regions that were active only in the highly tolerant mice (i.e. mice that received fentanyl in the fentanyl-associated context): the medial dorsal thalamus and the anterior cingulate cortex. Subsequent chemogenetic silencing studies confirmed this. These regions are known to be involved in the ascending pain pathway, as well as regulation of memory and environmental response.
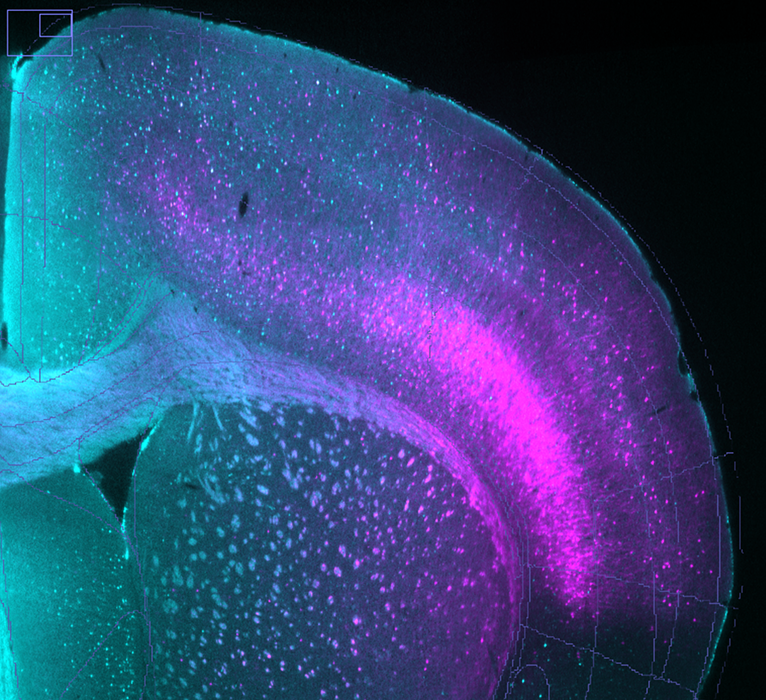
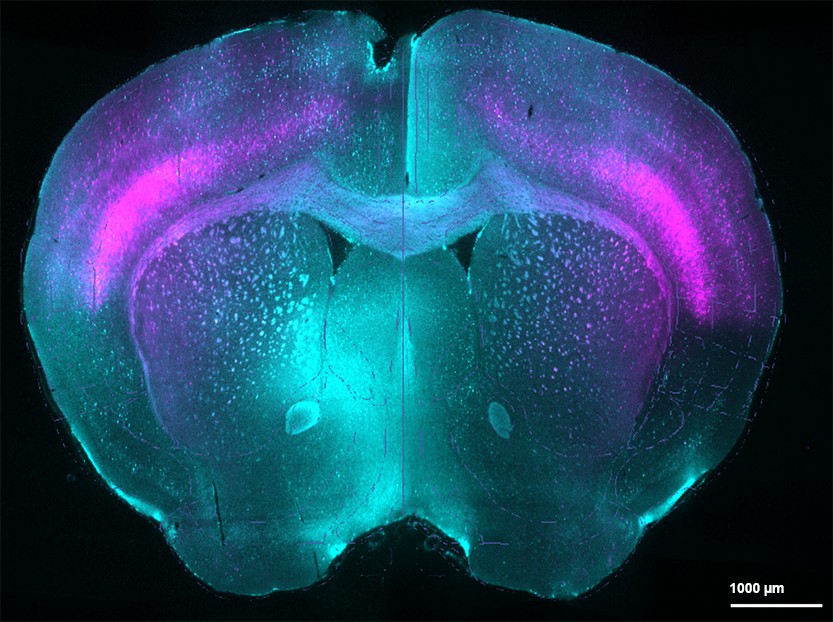
TdTomato-tagged cells (magenta) and c-FOS staining (cyan) in brain of fentanyl-tolerant mouse exposed to fentanyl-associated context.
However, we also saw something more striking: not only do tolerant mice have heightened activity in specific brain regions, tolerance-reversed mice (i.e. mice that received fentanyl in the saline-associated context) exhibit their own unique regional pattern of increased activity. These full-brain results correspond well with our IHC experiments, but they provide so much more information than IHC alone. We saw robust patterns of increased activity in areas that we wouldn’t have previously thought to explore, such as the brainstem and cerebellum.
In addition to the c-FOS immunolabeling panel, we also sent LifeCanvas brain samples from TRAP mice to look at specific ensembles of cells associated with tolerance. We identified and tdTomato-tagged cell ensembles that were active in fentanyl-tolerant mice, then one month later gave all of the mice fentanyl and separated them into two groups: half were placed in the banana room (fentanyl context, tolerant) and half in the almond room (saline context, tolerance-reversed). LifeCanvas sent us data on these tdTomato+ cells, as well as cells counter-stained for c-FOS, which we are currently analyzing in order to delineate these ensembles.
LC: What are the translational implications of these results?
"These full-brain results ... provide so much more information than IHC alone. We saw robust patterns of increased activity in areas that we wouldn’t have previously thought to explore, such as the brainstem and cerebellum."
RP: With this information, we can explore whether it’s possible to reverse tolerance of the analgesic effects of opioids, and consequently reduce opioid consumption, by modulating brain activity in specific regions. The traditional view of tolerance is that it’s defined by a series of fixed long-term biochemical and genetic adaptations, such as receptor desensitization and changes in the dorsal root ganglia.
But this work shows that it’s possible to eliminate some of the biological effects of tolerance almost instantly by administering the drug in a different environment. This gives me hope that there are alternative approaches for regulating tolerance that aren’t limited to exploring biochemistry or inherited genetics. Additionally, better understanding the contextual dependence of tolerance can help prevent overdoses, which can occur when an individual is not physiologically prepared for – or tolerant of – a high drug dose in a new environment.
LC: What are your future research plans?
RP: Now that we have narrowed down the brain areas that we believe are driving these context-dependent tolerance changes, we want to learn more about the molecular and genetic identities of the cells involved. Using approaches such as electrophysiology, fiber photometry, and genetics, we hope to find markers or druggable targets so that we can try to pharmacologically modulate tolerance. This is no trivial task! And while the medial dorsal thalamus and anterior cingulate cortex are our immediate areas of interest, there are still more areas to explore in depth.