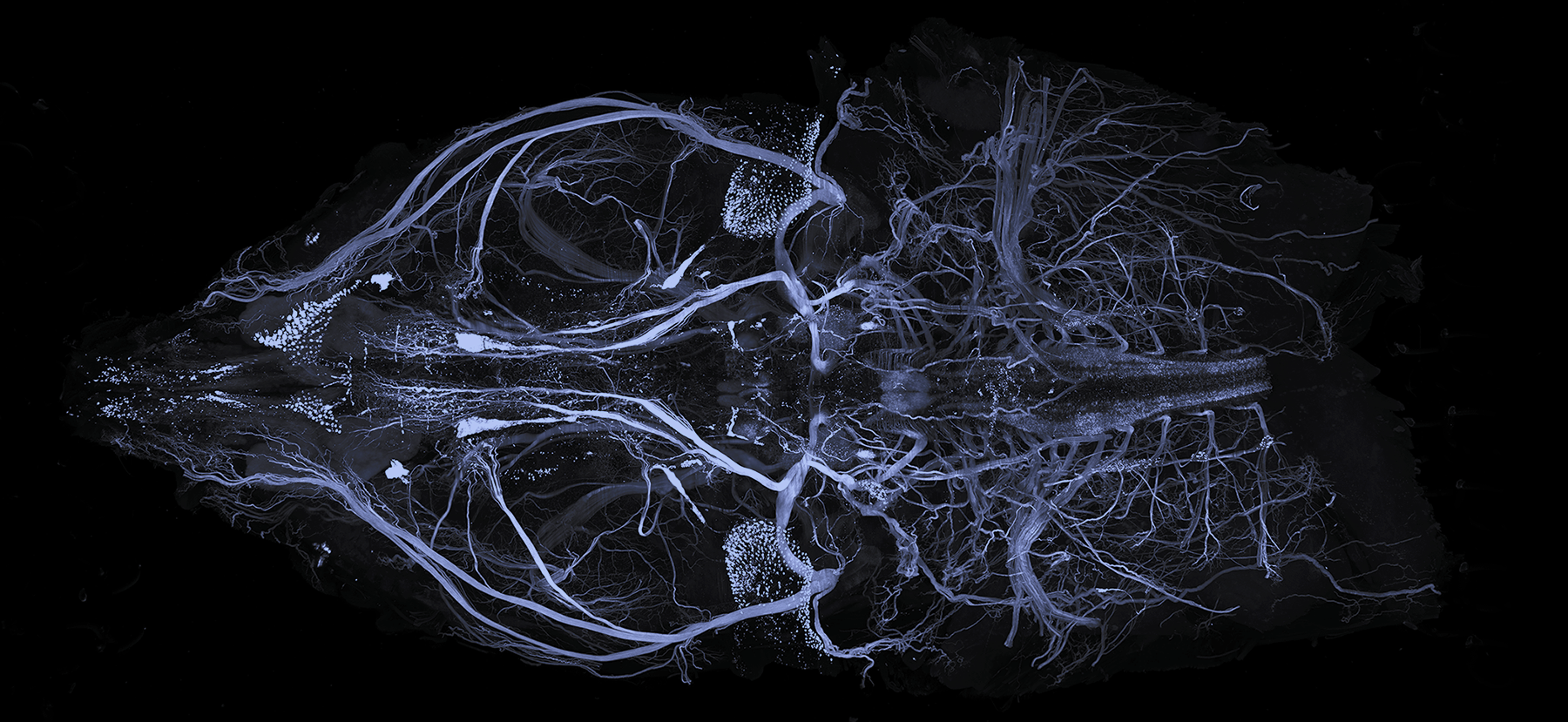
Whole Mouse Head Protocol
Reagents & Equipment Required
- 32% Paraformaldehyde Solution – (EMS) 15714-1L
- PBSN (PBS + 0.02% Sodium azide)
- Ethylenediaminetetraacetic acid (EDTA) – Sigma E9884
- Sodium hydroxide (NaOH) – Sigma 415413 (or any NaOH pellets)
- SHIELD Epoxy Solution (SH-ES) – Store at -20°C
- SHIELD Buffer Solution (SH-BS) – Store at RT
- SHIELD ON Buffer (SH-ON) – Store at 4°C
- Dichloromethane (270997, Sigma Aldrich)
- Tetrahydrofuran (186562, Sigma Aldrich)
- Quadrol (122262, Sigma Aldrich)
- Delipidation Buffer – stored at RT
- EasyIndex – RI = 1.52 – stored at RT in sealed container
- PBSTN (PBS + 1% TritonX-100 + 0.02% sodium azide)
- A shaking incubator capable of 4°C and 37°C
Perfusion
1. Transcardially perfuse the animal with ice-cold heparinized PBS. For mice, use about 20 mL and a 5 mL/min flow rate. For rats, use 200 mL and a 60 mL/min flow rate.
- We recommend using heparinized PBS to remove as much blood as possible (20 U/mL concentration).
- Perfuse with PBS until the fluid is running completely clear of blood before perfusing with ice-cold 4% PFA in PBS. Use the same volume and flow rates as before.
- Take extra care not to introduce air bubbles inside tubing.
- When the fluid comes out of the mouth or a lung swells, adjust the position of the needle in the heart.
- [Note] The better the perfusion, the better the results!
2. Remove the head. Careful dissection is essential to preserve the sample’s structural integrity.
3. Incubate the sample in 4% PFA in PBS for 24 hours at 4°C with shaking. If you are not ready to continue to the next step, the sample can be stored in 1X PBS with 0.02% sodium azide at 4°C until you are ready to continue.
Decalcification
1. Before proceeding, remove as much “extra” tissue from the sample as possible. It is best to remove skin or hair as much as possible – keratin does not clear very well for example.
2. Prepare a solution of 10% EDTA in distilled water. EDTA’s solubility is pH dependent, so add NaOH to dissolve the EDTA and adjust to pH ~7.5.
3. Incubate the sample in 10% EDTA at 4°C until the bone is soft and flexible. For a whole mouse head this takes 6 days.
SHIELD Fixation
Before proceeding, please check the Expiration Date on the SHIELD-Epoxy bottle. If the solution is used after the expiration date the mechanical stability of the sample can be compromised.
1. Prepare fresh SHIELD OFF Solution by mixing 20 mL SHIELD Epoxy Solution, 10 mL SHIELD Buffer Solution, and 10 mL distilled water.
2. Incubate the samples in SHIELD OFF Solution for 3 days at 4°C.
3. Incubate the samples in SHIELD ON Buffer for 24 hours at 37°C.
After this step you can store the samples in PBSN at 4°C until you are ready to proceed.
Organic Solvent Delipidation
Please note that dichloromethane and tetrahydrofuran are volatile organic solvents and should be handled with appropriate PPE in a fume hood. Samples should also be processed in polypropylene tubes as these chemicals can dissolve many plastics.
Also note, this protocol takes a full day for dehydration, another day in dichloromethane, and another full day to rehydrate, so plan ahead!
1. Prepare a solution that is 25% Quadrol, 75% PBS. We will call this solution PBSQ. Note that quadrol is very viscous. Once this solution is made, store it at 4°C.
2. Dehydrate the sample using a stepwise incubation of tetrahydrofuran (THF) and PBSQ. This should be done in 5 mL tubes filled all the way up. If the sample doesn’t fit in 5 mL tubes, larger tubes can be used but you will need to use more solution. Incubate the sample as follows:
- 50% THF, 50% PBSQ, 1.5 hours at 4°C.
- 70% THF, 30% PBSQ, 1.5 hours at 4°C.
- 80% THF, 20% PBSQ, 1.5 hours at 4°C.
- 95% THF, 5% PBSQ, 1.5 hours at 4°C.
- 95% THF, 5% PBSQ, 1.5 hours at 4°C.
- Dichloromethane, 30 minutes at 4°C.
- Refresh dichloromethane and incubate overnight at 4°C.
- In the morning, refresh dichloromethane, and incubate for 24 hours more at 4°C.
3. Once the sample has been dehydrated and treated with dichloromethane, it needs to be rehydrated. Incubate the samples in 5 mL tubes filled all the way up as follows:
- 95% THF, 5% PBSQ, 1.5 hours at 4°C.
- 95% THF, 5% PBSQ, 1.5 hours at 4°C.
- 80% THF, 20% PBSQ, 1.5 hours at 4°C.
- 70% THF, 30% PBSQ, 1.5 hours at 4°C.
- 50% THF, 50% PBSQ, 1.5 hours at 4°C.
4. Move the sample to PBSN, and wash for at least 24 hours at RT, with several PBSN refreshes before continuing.
Aqueous Delipidation
1. Incubate the samples in 40 mL Delipidation Buffer at 45°C for 10 days.
Optional Immunolabeling
Please note that this step is not fully tested or optimized, and that there may be significant limitations to labeling intact mouse heads Therefore, please treat this protocol as a starting point for future optimization and validation.
BLOCKING
1. Make 5 mL of 5% Donkey Serum in Antibody Blocking Solution per sample.
[Note] If you are using Goat secondaries, add 5% Goat serum to the Antibody Blocking Solution.
2. Incubate each sample in a 5 mL tube passively for 3 days at 37°C with shaking.
IMMUNOLABELING
1. Prepare enough staining solution in PBSTN to fully cover the sample. As a starting point, add 5-20 µg of each antibody. Add 250 µL normal serum to the solution.
2. Incubate in staining solution at 37°C for 10 days.
3. Wash in PBSTN for 24 hours at 37°C with several refreshes.
4. Fix the sample overnight in 4% PFA in PBSN at RT.
5. Wash out PFA with PBSTN over 8 hours at 37°C with several refreshes.
6. Prepare secondary staining solutions in the same volume of PBSTN. Add secondaries in a 2:1 molar ratio to the primaries. Add 250 µL normal serum to the solution. The 647 nm channel is best, with 561 nm as second best for large tissues.
7. Incubate in secondary staining solution at 37°C for 10 days.
8. Wash in PBSTN for 24 hours at 37°C with several refreshes.
9. Fix the sample overnight in 4% PFA in PBSN at RT.
10. Wash out PFA with PBSN over 8 hours at 37°C with several refreshes.
Index Matching
1. Prepare a solution of 50% EasyIndex in distilled water. Incubate the samples for 24 hours at 37 °C.
2. Move the samples to 100% EasyIndex and incubate for 24 hours at 37°C or until transparent. If there is a significant amount of tissue around the bone, this can take more than 24 hours to index match.
[Note] Bubbles may form inside the sample and become visible after index matching. These bubbles can interfere with imaging. The best way to remove them is with a small gauge needle. Put the sample in a petri dish filled with EasyIndex and use the needle to withdraw the bubbles inside the sample. It’s important to keep the sample submerged while removing the bubble so the air gap can be filled with EasyIndex and won’t fill back up with air.
3. Mount the sample as normal!
References
This protocol is based on an adaptation of the HYBRiD method from Victoria Nudell et al: Nudell et al., (2022), Nature Methods. 479–485
