Standard SHIELD Protocol
After perfusion, the sample will be incubated in SHIELD OFF Solution to allow epoxy to diffuse into the sample. Finally, the epoxy will be crosslinked during the SHIELD ON step. We recommend starting SHIELD on a Friday or a Monday to avoid work on the weekends.
[Note] If you store the SHIELD-Epoxy in the freezer, you will need to take it out before performing SHIELD to thaw. Alternatively you can aliquot some in advance and store it in the fridge as a shorter term storage option.
Reagents/Equipment Required
- SHIELD Epoxy Solution (SH-ES) – Store at -20°C
- SHIELD Buffer Solution (SH-BS) – Store at RT
- SHIELD ON Buffer (SH-ON) – Store at 4°C
- Temperature controlled shaking incubator capable of 37-45°C
Protocol
Before proceeding, please check the Expiration Date on the SHIELD-Epoxy bottle. If the solution is used after the expiration date the mechanical stability of the sample can be compromised.
[Note] Once you begin the first SHIELD step, do not stop the process until SHIELD fixation is completed so make sure to plan ahead.
SHIELD OFF
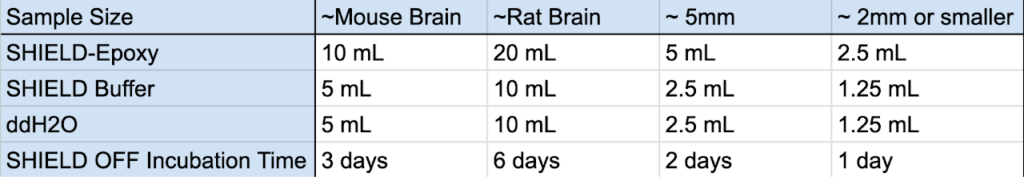
1. Prepare fresh SHIELD OFF Solution. Mix the solutions in the below ratios.
- SHIELD-OFF Solution: (by volume) 50% SHIELD-Epoxy, 25% SHIELD Buffer, 25% water
- See the recommended SHIELD-OFF solution amounts for different samples in the table below.
2. Incubate the samples in SHIELD OFF Solution for the times indicated in the table at below 4°C with light shaking.
SHIELD ON
If your sample’s smallest dimension is 1.5 mm or smaller, please stop here after SHIELD OFF incubation and continue to step 3 of Small Sample SHIELD protocol.
3. Incubate the sample in SHIELD ON for 24 hours at 37°C with light shaking. Mouse brains will require 20 mL while rat brains require 40 mL.
SHIELD preservation is now complete. The sample can be stored in 1X PBS with 0.02% sodium azide at 4°C for several months without significant loss of fluorescence signal and structural integrity.
You may now continue on to Delipidation.
