Passive Labeling
To increase penetration of antibodies into large, intact tissues, we recommend using active staining SmartLabel or SmartBatch+. You can proceed with normal IHC protocols but with extended staining durations and increased antibody amounts as needed. This optimization is highly empirical and variable from antibody to antibody. In some cases, it may not be possible to achieve uniform staining passively. If your samples are small (50-200 µm slices), normal IHC protocols as recommended by antibody manufacturers can be followed.
Suggestions
BLOCKING
An additional blocking step before labeling may be helpful, especially to prevent nonspecific binding. Whether or not this step is needed will depend on the antibody and tissue, and can be determined experimentally.
Protocol:
- Make 5 mL of 5% Donkey Serum in Antibody Blocking Solution per sample.
- [Note] If you are using Goat secondaries, add 5% Goat serum to the Antibody Blocking Solution.
- Incubate each sample in a 5 mL tube passively for 2 days at 37°C with shaking.
ANTIBODY FIXATION
An additional PFA fixation step after primary and secondary labeling may be helpful.
- PFA fixation after primary can help reduce aggregate-like nonspecific signal.
- PFA fixation after secondary will prevent potential dissociation of antibodies in EasyIndex.
- After washing, incubate the sample in 4% PFA in 1X PBS overnight at RT with shaking.
CHANNELS
Due to native autofluorescence of the tissue, the lower wavelengths may be less useful. In our hands, the 647 channel is best, followed by 561. 488 can be useful depending on the target and your application since autofluorescence can overpower the signal. In the sample below, we labeled a whole brain with anti-calretinin antibody. Then we stained with 3 secondaries at the same time, in the 488, 561, and 647 channels using equal concentrations of secondary. In 488, you can really only see cell bodies. In 561 you can see cell bodies and some of the brighter fibers. In 647 you can see cell bodies and all of the dimmer fibers.
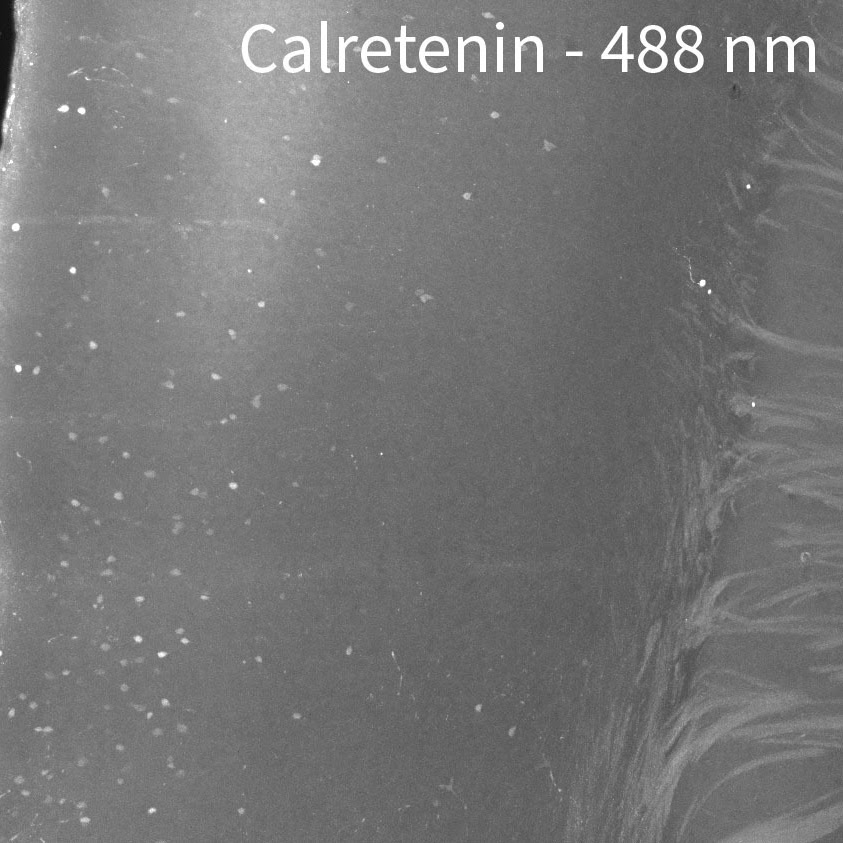
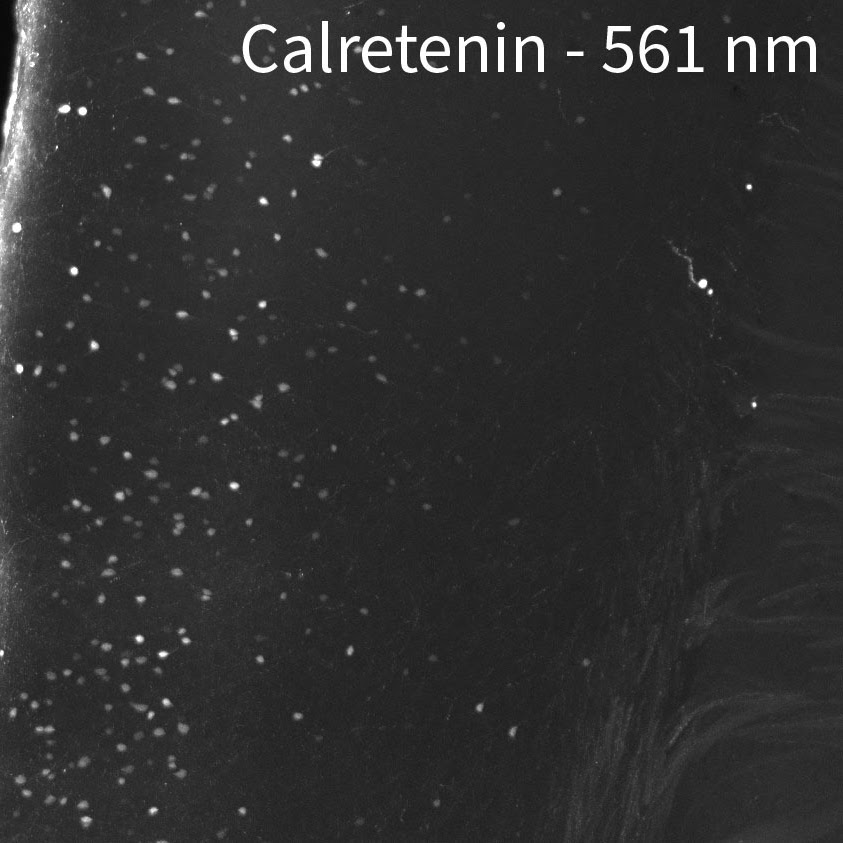
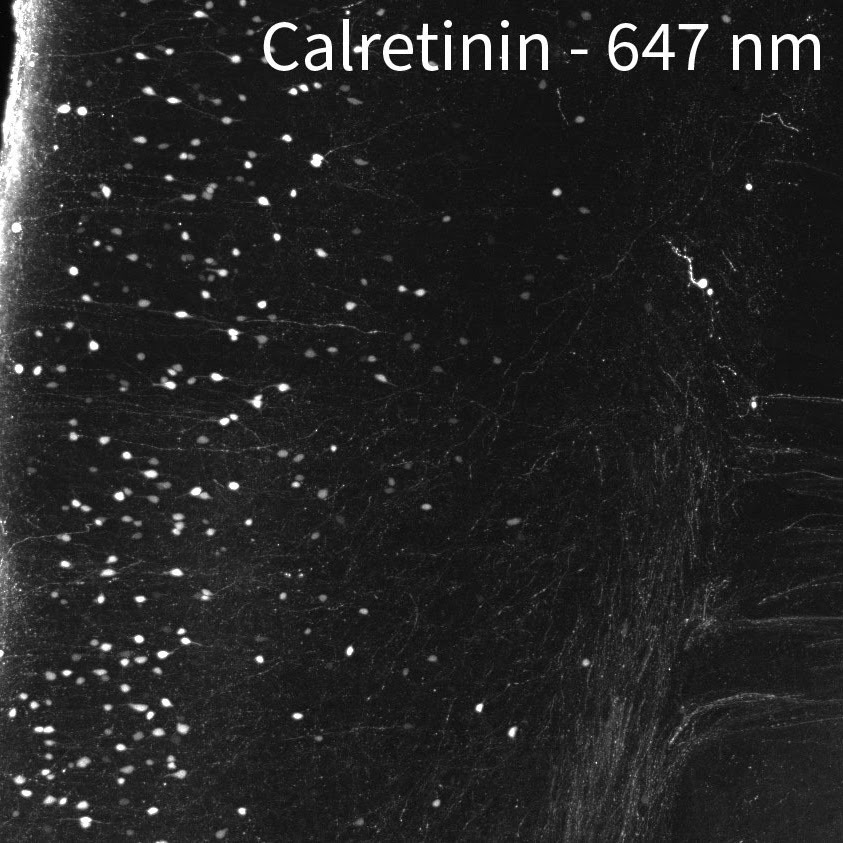
If you are interested in FISH, please consult the original SHIELD manuscript or contact the authors for more information.
If you would like to validate an antibody not on the validated antibody page, follow this protocol.
If you validate a new antibody, please let us know at info@lifecanvastech.com and we can provide a discount on a reagent purchase!
