Active Labeling with SmartLabel
Choosing appropriate antibodies and dyes
The protocol can accommodate:
- Staining with dye-conjugated antibodies
- Simultaneous delivery of primary along with monovalent Fab fragment secondaries
- Sequential delivery of primary and secondary (can be Fab or IgG)
Please consult the Validated Antibody List for more information as it can take significant time and work to validate and optimize antibodies. We have also found that some antibodies require specific secondary delivery schemes, or are only bright enough to work in certain channels.
Are you using an antibody for the first time?
We recommend first following the Antibody Validation Protocol. If you wish to skip that step, we recommend using a sequential delivery of primary and secondary for the first test since simultaneous Fab fragments can change the binding affinity of the primary antibody.
Additional notes and recommendations:
- If you anticipate a signal being dim, we recommend sequential delivery of secondary using whole IgG secondaries in the 647 nm channel.
- Make sure you avoid antibody host conflicts with primary antibodies and wavelength conflicts with secondary antibodies.
- If you are delivering a nuclear dye or vasculature stain, please add them during this primary step. In general, we recommend using higher wavelengths (red or far red) for antibodies and saving lower wavelengths for brighter targets or nuclear dyes.
- If you are using a small molecular dye, do so during primary labeling. To prevent the cup from being contaminated with the dye, use the same cup during secondary staining and post wash. This pulls any dye that got stuck in the cup during primary labeling back out of the cup. If you do not use the same cup in secondary labeling, quarantine the cup to use only with other samples being stained with the same dye.
Note regarding using both chambers of the SmartLabel at the same time:
- It is very common to use both the right and left chambers of the SmartLabel to run labeling simultaneously.
- These two chambers have isolated device buffers and regions for samples cups, so you may use different antibodies on each side.
- However, the measured current that is displayed on the device screen is the summation of the individual currents from each chamber. Additionally, each chamber will receive the same amount of voltage. Therefore, it is not advisable to run primary labeling in one chamber and secondary in the other.
- If you wish to use just one side of the SmartLabel, turn off the pump on the unused side.
Reagents Required
- SmartLabel
- SmartLabel Reagents – check bottles for storage conditions.
- SmartLabel Primary Sample Buffer
- SmartLabel Primary Device Buffer
- Secondary Sample Buffer
- SmartLabel Secondary Device Buffer
- Antibody Blocking Solution
- PBS with 0.02% sodium azide (PBSN)
- Donkey serum
- Labeling Reagents, such as primary and secondary antibodies or fluorescent nuclear dyes. Please consult Validated Antibody List for more information.
- Single Staining Cup
- Mesh bag inserts
- Sample cup storage solution. More can be made fresh here.
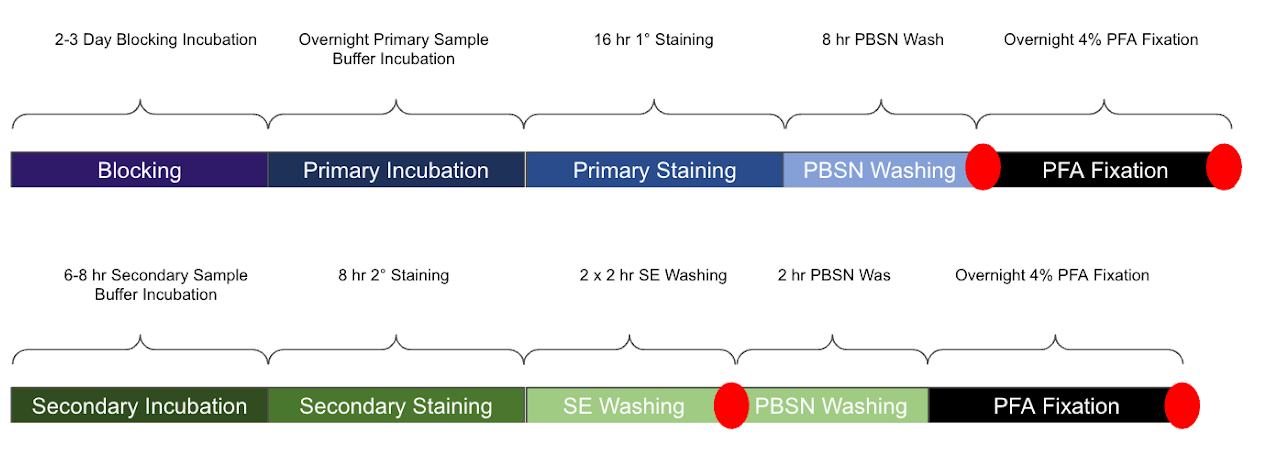
Refer to the timeline above. The red dots indicate optional stopping points. If you are not ready to move on to the next steps, move the samples to PBSN at 4°C until you are ready to proceed.
Protocols
BLOCKING
[Note] Blocking may not be needed for some antibodies. This can be determined experimentally. We know it is essential for active labeling with some antibodies such as c-fos.
Preparation Days
1. Make 5 mL of 5% Donkey Serum in Antibody Blocking Solution per sample.
- Incubate each sample in a 5 mL tube passively for 2 days at 37°C with shaking.
- [Tip] You may block for up to 3 days. 3-day blocking will avoid steps on the weekend. See the schedule suggestion for more tips.
- If you are going to be using Goat secondaries, add 5% Goat Serum in Antibody Blocking Solution instead.
PRIMARY LABELING
Day 1
1. Pre-incubate in SmartLabel Primary Sample Buffer. Start pre-incubating 1-3 days before you plan to start labeling.
- Fill a conical tube with 20 mL of SmartLabel Primary Sample Buffer.
- Place the sample in the tube, covered from light and shake at room temperature.
Day 2
1. Refresh the SmartLabel Primary Sample Buffer the next morning.
- Allow the sample to shake in the refreshed buffer for at least 3 hours.
2. Around 3 pm, prepare the cup and device for primary labeling.
Cup preparation
[Note] Only Single Sample Cups are compatible with SmartLabel.
[Note] Primary labeling runs in the SmartLabel+ for 16 hours. You want to start labeling later in the work day so that the samples are not sitting in the device for too long after finishing the next morning.
- Remove the Single Staining Sample Cup from its storage solution.
- Use a very gentle stream of tap water to carefully rinse the cup, holding the cup close to the tap. The cups are fragile so do not subject them to high water pressure.
- Once rinsed out with tap water, carefully rinse the cup with distilled water.
- Gently dry the cup with a clean paper towel or kim wipe, making sure to not apply too much pressure to the membranes.
- Place the cup on a fresh paper towel and fill it to the top with DI water. Let this sit here for several minutes, checking on the paper towel surrounding the cup for leaks. Check out the leak testing page for more information.
Device preparation
- While the cup is leak testing, wash the device and soak up any remaining liquid in the Chamber with paper towels.
- Ensure the Drainage Valve is closed and add 300 mL of SmartLabel Primary Device Buffer into each chamber you will be using. This is an entire bottle.
- [Note] If you are using both chambers, pour 300 mL of the buffer into each chamber.
3. Prepare samples and solutions.
Load the sample buffer into the cup
- When you are sure the cup is not leaking, pour a small amount of SmartLabel Primary Sample Buffer into the cup and swirl it around to coat the membrane. Dump and discard this liquid. Repeat this a second time.
- Fill the sample cup with 9 mL of SmartLabel Primary Sample Buffer.
Add antibodies / dyes to the Sample Cup
[Note] If you are going to use whole IgG secondaries, they cannot be added during this step and must be delivered sequentially. If you are going to use simultaneous Fab fragment secondaries, you should add them to the cup, generally in a 2:1 molar ratio. Please note that IgG antibodies have a MW of 150 kDa, while monovalent Fab fragments have a MW of 50 kDa.
- Add the appropriate µgs of antibody to the cup. Consult the Validated Antibody List for recommended amounts of antibodies to use.
- Add Normal Serum.
- Use Normal Donkey Serum if you will use donkey host secondaries, and Normal Goat Serum for goat host secondaries.
- Single Sample Staining Cup: 200 µL serum
- Use Normal Donkey Serum if you will use donkey host secondaries, and Normal Goat Serum for goat host secondaries.
Mix the liquid in the cup well using a fresh pipette.
Load the samples into the cup.
- If you have not already, place each sample in a mesh bag, keeping track of which samples is in which bag (if you are using both chambers).
- One mesh bag can hold one brain.
- Place the sample in the mesh bag and the mesh bag into the single cup.
- Look at the samples inside the cup from the side through the membrane. If you see any bubbles between the bag and the sample, you can lower a pipette or small spatula into the cup to remove the bubble.
4. Load the cup into the device and start the experiment.
- Place the sample cup into the Chamber. Line up the hex on the bottom of the cup with the hex in the Chamber.
- Turn on the pumps for the chambers you are using.
- Turn on stirring, you may be able to hear and or see the stir bar in the cup rotate.
- Turn on the Rotation.
- Close the Chamber Lid and Case Lid.
- Confirm the following settings:
- 30°C, 90V, and 500 mA current limit per chamber.
- If you are using both chambers, set the current limit to 1000 mA and make sure the leads are plugged into both chambers.
- If you are using one chamber, set the current limit to 500 mA and ensure the pump is turned off in the chamber you are not using.
- Change the timer to 16 hours.
- 30°C, 90V, and 500 mA current limit per chamber.
- Turn on Electrophoresis and Timed Shutdown.
- The timer will automatically turn off electrophoresis at the end of the experiment.
- These are normal starting values for current and voltage (They can vary more depending on temperature):
- Current: 300-350 mA per chamber
- Voltage: 90V
PRIMARY WASHING AND FIXING
Day 3
1. Check to see if the experiment is complete.
- Use a pipette to remove ~20 µL of solution from the Sample Cup and pipette it onto a pH paper strip.
- If the pH is still above ~8.3, you will need to extend the duration of the experiment to complete antibody binding.
- For every 0.1 above pH 8.3, the experiment should run for an additional hour. So if you measure a pH of 8.5, change the timer to 2:00 (2 hours) and turn on Electrophoresis Power and Timed Shutdown.
- When the pH has dropped below 8.3 and labeling is complete, open the device and remove the Sample Cup.
2. Move the samples to PBSN.
- Prepare a conical tube with ~40 mL of PBSN.
- Remove the mesh bag from the Sample Cup and place it in the tube.
- Wash the sample until the end of the day in PBSN at RT with light shaking, refreshing the solution at least once. If your sample contains fluorescent signals, protect from light.
3. Clean the device and cup.
- Carefully rinse the Sample Cup with distilled water and store it in its storage solution. It is important to keep the membrane hydrated at all times. We recommend refreshing the storage solution every few months. More can be made here.
- Wash the device before shutting it down.
4. PFA fixation.
[Note] Whether you need to add sequential secondaries or not, we recommend PFA fixing the samples at this point to prevent antibody dissociation. If you are not ready to do an overnight PFA fix, simply keep the samples in PBSN at 4°C until you are ready. Extended time before fixing can result in antibody dissociation from epitope binding sites.
- At the end of the day, prepare 20 mL of 4% PFA in 1X PBS in a falcon tube and move the sample to this tube.
Incubate samples overnight at RT with light shaking protected from light.
SECONDARY BUFFER INCUBATION AND SEQUENTIAL SECONDARY LABELING
Day 4
If you are ready to complete secondary staining in this day (immediately after PFA fixation), you can immediately continue to the next step. Otherwise, move the sample to PBSN and store it at 4°C protected from light until you are ready.
1. The next morning start secondary incubation at 37°C.
[Note] You do not need to PBSN washed between PFA and secondary sample buffer because the buffer contains tris which neutralizes the PFA.
- Move the sample to a fresh tube of ~20 mL of Secondary Sample Buffer.
- Incubate samples for 6-8 hours at 37°C with light shaking, refreshing the solution once halfway through the incubation.
2. Around 4 pm, prepare the cup and device for secondary labeling.
Cup preparation:
- Wash and leak test a Single Sample Cup the same way you did in primary labeling.
Device Preparation:
- Wash and dry the device.
- Ensure the drainage valve is closed and add 300 mL of Secondary Device Buffer into each chamber you will be using .
3. Prepare samples and solutions.
Load the sample buffer and samples into the cup.
- When you are sure the cup is not leaking, pour a small amount of Secondary Sample Buffer into the cup and swirl it around to coat the membrane. Dump and discard the liquid in the cup. Repeat a second time.
- Fill the Single Sample Cup with 9 mL of Secondary Sample Buffer.
Add secondary antibodies and dyes as needed to the Sample cup
- Check the validated antibody page to see what molar ratio between primaries and secondaries you should be using.
- For most but not all antibodies, 2:1 is the molar ratio recommended for Secondary:Primary. Check the Validated Antibody list for more information.
- Add Normal Serum (Optional).
- Use Normal Donkey Serum if you will use donkey host secondaries, and Normal Goat Serum for goat host secondaries.
- Single Sample Staining Cup: 200 µL serum
- Use Normal Donkey Serum if you will use donkey host secondaries, and Normal Goat Serum for goat host secondaries.
Load the samples into the cup.
- If you have not already, place each sample in mesh bag, keeping track of which samples is where.
- One mesh bag can hold one brain.
- One sample can go in each single sample cup.
- Look at the samples inside the cup from the side through the membrane. If you see any bubbles between the bag and the sample, you can lower a pipette or small spatula into the cup to remove the bubble.
4. Load the cup into the device and start the experiment.
- Place the sample cup into the Chamber. Line up the hex on the bottom of the cup with the hex in the Chamber.
- Turn on the pumps for the chambers you are using.
- Turn on stirring, you may be able to hear and or see the stir bar in the cup rotate.
- Turn on the Rotation. Check rotation is working.
- Confirm the following settings:
- 30°C, 90V, and 600 mA current limit per chamber
- If you are using both chambers, set the current limit to 1200 mA and make sure the leads are plugged into both chambers.
- If you are using one chamber, set the current limit to 600 mA and ensure the pump is turned off in the chamber you are not using.
- Change the timer to 8 hours.
- 30°C, 90V, and 600 mA current limit per chamber
- Turn on Electrophoresis and Timed Shutdown.
- The timer will automatically turn off electrophoresis at the end of the experiment.
- These are normal starting values for current and voltage (They can vary more depending on temperature):
- Current: 600 mA / chamber
- Voltage: 40-55 mA
POST-SECONDARY ELECTROPHORETIC WASH AND PFA FIXING
Day 5
1. When the experiment is over, start the post secondary electrophoretic wash.
[Note] There will be no pH drop during secondary labeling, so there is no need to check the pH.
[Note] There is no need to replace the secondary device buffer for the post wash.
- Open the device and remove the Sample Cup.
- Remove the samples from the cup.
- Pour out the antibody cocktail from the sample cup.
- Fill the cup with a small amount of Secondary Sample Buffer, swish it around gently and discard it. Repeat this a second time.
- Fill the cup again with the appropriate amount of Secondary Sample Buffer depending on cup size and place the samples back into the cup.
- Place the sample cup back into the device, lining up the hex pieces.
- Turn on the the pumps, stirring, and rotation. Check rotation is working.
- Keep the current, voltage, and temperature the same.
- Change the timer to 2:00 (2 hours).
- Turn on electrophoresis and start the timer.
Repeat this 2 hour wash once more, for a total of 6 hours in the post secondary electrophoretic wash.
2. Move the samples to PBSN.
- Prepare a conical tube with ~40 mL of PBSN.
- Then remove the mesh bag from the Sample Cup and place it in the tube.
- Wash the sample until the end of the day in PBSN at RT with light shaking, refreshing the solution at least once. If your sample contains fluorescent signals, protect from light.
3. Clean the device and cup.
- Carefully rinse the Sample Cup with distilled water and store it in its storage solution. It is important to keep the membrane hydrated at all times. We recommend refreshing the storage solution every few months. More can be made here.
- Wash the device before shutting it down.
4. PFA fixation.
[Note] The secondary antibodies will now be fixed at the end of the day. If you are not ready to do an overnight PFA fix, simply keep the samples in PBSN at 4°C until you are ready. Extended time before fixing can result in antibody dissociation from epitope binding sites.
- At the end of the day, prepare 20 mL of 4% PFA in 1X PBS.
- Move the sample from PBSN to a fresh tube of 4% PFA. The samples can remain in their mesh bags.
Incubate samples overnight at RT with light shaking protected from light.
Day 6
1. The next morning transfer the samples to 40 mL of PBSN to wash out the PFA.
- Wash for at least 6 hours, refreshing the PBSN once halfway through.
You have completed Immunolabeling. Move onto Index Matching.
