April is National Inventor’s Month, and we wanted to give a shoutout to historical inventors that had a hand in creating the technologies we use every day in our manufacturing and tissue processing lab. Advances in science are incredibly interconnected, requiring the use of materials and equipment from multiple fields with diverse contributors and inventors. Below are a few of our favorites:
Richard Zsigmondy
Inventor of the ultramicroscope, ancestor of modern light sheet microscopy
Zsigmondy was an Austrian chemist whose work with colloids (in particular, colored glass) led him to develop a method of illuminating particles too small to be detected by contemporary methods of light microscopy. He and Henry Siedentopf published this visualization method in 1903 and called it the ultramicroscope. The apparatus used sunlight passing through a slit aperture as the light source and placed the objective at a right angle to the angle of illumination. Zsigmondy would later win a Nobel Prize in chemistry for his research on colloids and the ultramicroscope.
Modern light sheet microscopy, though not developed until the late 20th century, is based on Zsigmondy’s research. Our SmartSPIM light sheet microscope uses precision lasers instead of sunlight and an index matching immersion chamber instead of air. Nevertheless, the advanced technology that we now use to probe the complexities of the brain is descended from these basic methods. The light sheet community owes its foundation to a century-old invention by a chemist studying rose-colored glass.
Fun fact: Though cranberry glass is believed to be produced as far back as the 4th century, Zsigmondy was the first person to describe the mechanism for how the color is produced. The secret is the addition of gold chloride to the molten glass.
Albert Coons
Developer of the field of immunofluorescence
Albert Coons was an American physician, immunologist, and professor at Harvard Medical School who developed the first technique for attaching fluorescent molecules to antibodies. During a vacation in Germany in 1939, Coons had an epiphany that led to an extensive project in collaboration with researchers at Harvard University to label antibodies with fluorescent dyes. Importantly, Coons was able to demonstrate that labeling the antibodies did not affect their binding affinity or specificity for their target antigens (J Immunol, 1942). Fluorescent antibodies are an indispensable tool in laboratories all over the world and make up a large portion of the multi-billion-dollar antibody industry.
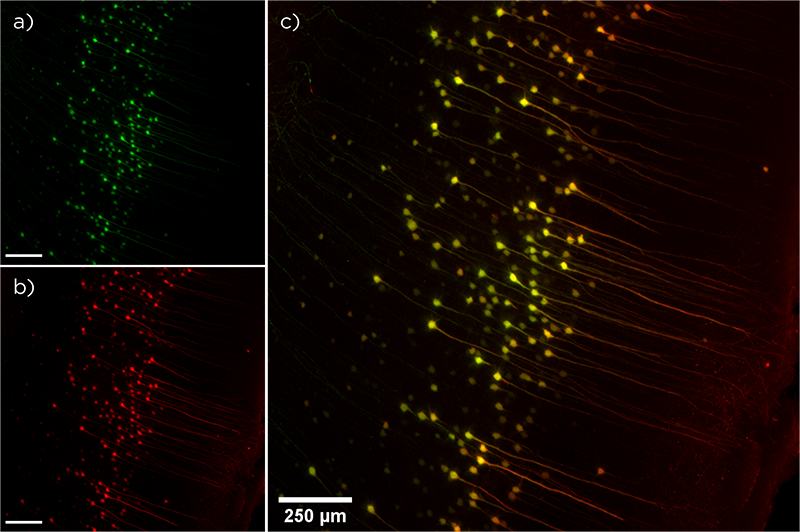
The fluorescent antibodies LifeCanvas uses every day in our research laboratory are direct descendants of Coons’ invention. Indeed, part of our tissue processing pipeline relies on immunofluorescence technology to visualize protein expression and localization throughout whole organs. Our tissue clearing and immunolabeling device SmartBatch+ distributes fluorescent antibodies that can then be imaged with our light sheet fluorescence microscope, SmartSPIM. Labeling with immunofluorescent antibodies complements other biological visualization techniques such as injection of a fluorescent dye or fluorescent molecule-expressing virus, or insertion of a fluorescent molecule into a protein’s genome using the CRISPR-Cas9 technique.
Arne Tiselius
Inventor of electrophoresis
Electrophoresis is widely employed in research to manipulate migration of molecules based on their electrical properties. The method was invented by Arne Tiselius, a Swedish biochemist, while working on his doctorate at the University of Uppsala. He was awarded the Nobel Prize in chemistry in 1948 for his work on adsorption and electrophoresis. The first generation of electrophoresis was called “moving boundary electrophoresis,” and used a simple U-shaped glass tube filled with a buffer solution with a positive electrode at one end and a negative electrode at the other. Molecules (generally proteins or nucleic acids) migrated to one side of the tube or the other depending on their charge and thereby formed bands within the solution.
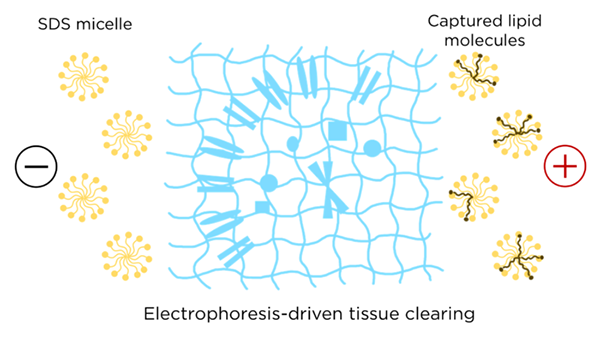
Electrophoresis is still commonly used in research and manufacturing labs to separate biomolecules or other charged particles based on molecular weight and charge. At LifeCanvas, electrophoresis is a key technology behind stochastic electrotransport in our SmartBatch+ device, driving charged surfactant micelles deep into biological tissue to extract lipid molecules.
Electrophoresis is also at work in SmartBatch+‘s labeling function, where antibodies are distributed throughout intact tissues by using an electric field. Whole organ clearing and immunolabeling at the current level of speed and uniformity would not be possible without Tiselius’ invention!
Fun fact: You can make an inexpensive gel electrophoresis kit at home using household materials like aluminum foil, agar gelatin powder, and lemonade.
Heinrich Schnitger
Inventor of the micropipette
The micropipette has been an essential lab tool for decades. It was first patented by Heinrich Schnitger while working as a postdoc at the University of Marburg, Germany in 1957. Previous methods of transferring small amounts of fluid involved “mouth pipetting,” the process of drawing fluid up into a glass pipette using mouth suction and then expelling it. Schnitger’s goal was to develop a method of handling small volumes of fluid with speed and precision (avoiding being poisoned or infected was a nice bonus). His invention included a spring-loaded piston, a replaceable plastic tip, and an air buffer between the tip and the piston common to all modern versions of the micropipette.

LifeCanvas researchers use micropipettes every day in the lab to handle reagents such as antibodies, SmartBatch+ staining buffer, and EasyIndex, our refractive index matching solution. Few lab tools are as ubiquitous and relatively unchanged from their original versions as the micropipette.
Fun fact: The first recorded infection attributed to mouth pipetting occurred in 1893, when a physician accidentally ingested a culture of typhoid bacilli. Remarkably the practice was still commonplace until the 1970s, when cheap, adjustable micropipettes became commercially available.