Spotlight on Innovation: Tanya Daigle's, Award-Winning Spinal Cord Video, imaged with SmartSPIM:
We’re excited to spotlight Tanya Daigle from the Allen Institute, whose award-winning video of a mouse spinal cord, imaged with our SmartSPIM Light Sheet Microscope, won second place in the 2024 BRAIN Initiative Photo and Video. In this interview, Daigle shares insights into her research and how our 3D imaging has been crucial to her discoveries.
LC: Can you tell us about your journey and what you are currently researching?
TD: I joined the Allen Institute in 2013 and now lead a team within the Human Cell Types department, focusing on genetic tool development for both basic and translational applications. My academic background includes a B.S. from U.C. San Diego and a Ph.D. in Physiology and Biophysics from the University of Washington. My career path has taken me through research positions at the Salk Institute, Duke University, and the McGovern Institute for Brain Research at MIT. As a molecular neuroscientist, my expertise lies in genetic tool development, neurodegenerative disorders, addiction-related disorders, and human gene therapy.
At the Allen Institute, I’ve dedicated the past decade to creating innovative tools that advance our understanding of cellular mechanisms and their impact on higher-level brain functions. Recently, my focus has expanded to applying these tools to human diseases, particularly neurodegenerative conditions and disorders involving the dopamine system and basal ganglia, like Parkinson’s disease.
LC: What has been your experience with 2D and 3D? What are the advantages of transitioning to 3D Imaging?
"Transitioning from 2D imaging to 3D volumetric approaches has transformed our understanding of biological structures."
TD: Transitioning from 2D imaging to 3D volumetric approaches has transformed our understanding of biological structures. While 2D techniques like epifluorescence and confocal microscopy are useful, they have limitations such as incomplete views of complex systems. LifeCanvas Technologies’ 3D imaging offers a holistic view, revealing unprecedented details and insights. This shift accelerates research by providing faster and more comprehensive data acquisition.
"LifeCanvas' 3D imaging offers a holistic view, revealing unprecendented details and insights. This shift accelerates research by providing faster and more comprehensive data acquisition"
LC: Do you see your work being applied only for research, or do you think it has therapeutic potential as well?
TD: Absolutely, our work extends far beyond research into precision therapeutics. We believe that the technology we’re developing can revolutionize how we approach treatment. At the Allen Institute, we’re pioneering methods that allow us to precisely target any cell type in the brain and body, which opens up incredible possibilities for delivering therapeutic agents or even performing gene editing. Currently, we have an active human gene therapy program and have partnered with a Pharmaceutical to conduct preclinical research. These efforts are focused on exploring and validating the potential of our technology to treat human diseases. We’re excited about the future and our role in advancing these groundbreaking therapeutic applications.
LC: How do you develop these viruses, and why are they important?
TD: We use single-cell genomics, like RNA sequencing, to identify gene regulatory enhancers. These enhancers are cloned into viral vectors and tested in mice through IV or retro-orbital injections to observe expression patterns. Initially, we use a fluorescent protein, SYFP2, to confirm cell type-specific activity, crucial for developing our spinal motor neuron-targeting virus.
This virus is essential for studying neurodegenerative diseases like ALS and SMA, as it specifically targets spinal motor neurons, which current viral vectors cannot do effectively. Tested in macaques, our virus shows strong expression in the spinal cord, offering potential for targeted gene therapy and more effective treatments for ALS and SMA.
LC: How has our technology, including 3D Imaging with SmartSPIM, helped your research?
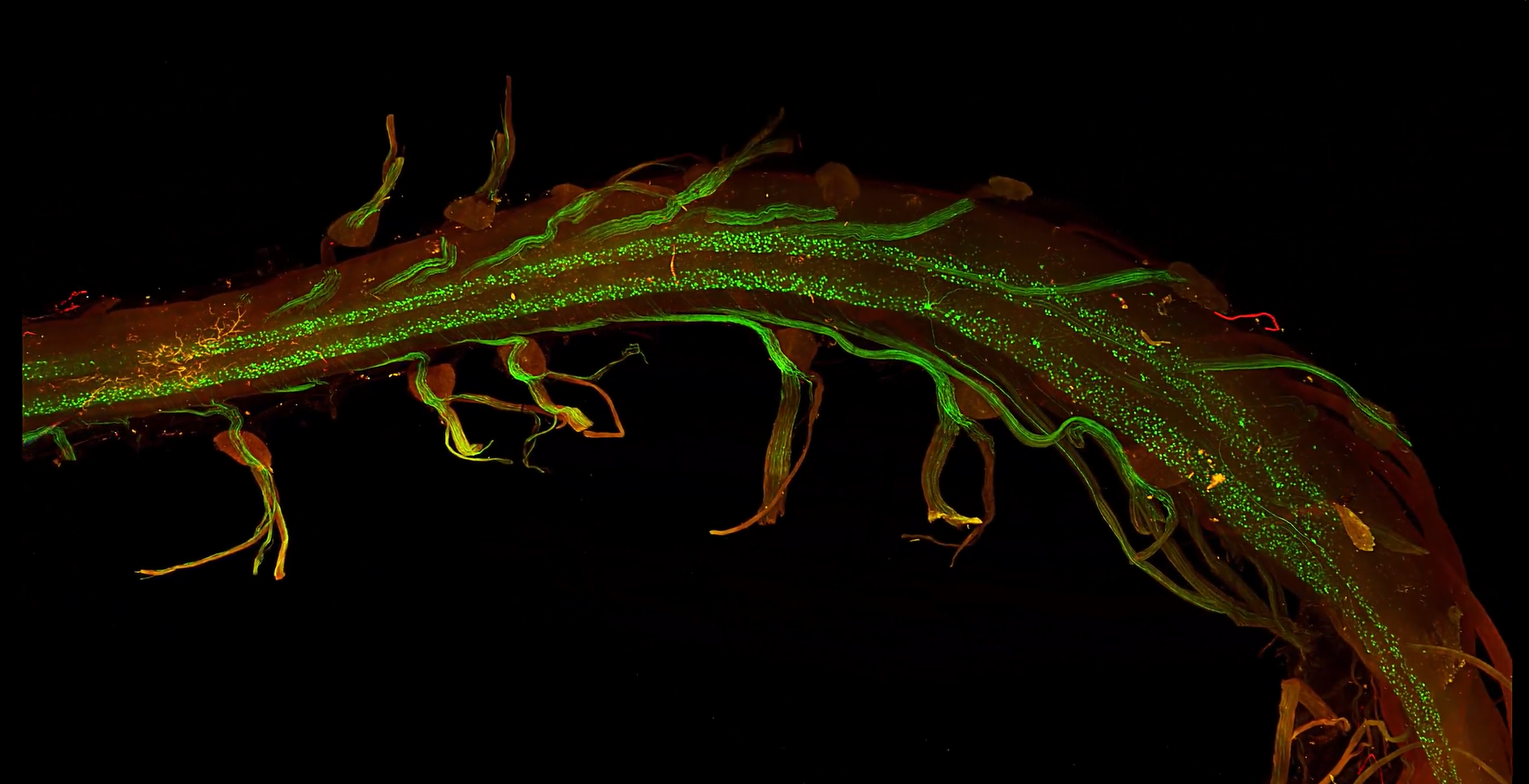
TD: One of the major challenges in our research was imaging the dorsal root ganglia (DRG’s) associated with the spinal cord. Motor neurons, though fewer in number compared to other neuronal populations, extend large, branching axons that relay signals from the brain to the muscles. Traditional 2D imaging provided only fragmented glimpses of these intricate structures, showing isolated segments without capturing their full scope. LifeCanvas’ SmartSPIM technology transformed this process, revealing the beautiful curvature of axons around the DRGs, a view we had never fully appreciated before. This advancement was crucial not only for validating our tools but also for deepening our understanding of neuronal function and interaction. This technology eliminated the need for mental reconstruction and inference, reducing the risk of inaccuracies and enhancing our comprehension of these complex neural structures.
"One of the major challenges in our research was imaging the dorsal root ganglia (DRG’s) associated with the spinal cord...Traditional 2D imaging provided only fragmented glimpses of these intricate structures, showing isolated segments without capturing their full scope. LifeCanvas' SmartSPIM technology transformed this process, revealing the beautiful curvature of axons around the DRGs, a view we had never fully appreciated before."
LC: What would you highlight about your collaboration with LifeCanvas?
"The quality and clarity of these visuals have been transformative for our research, providing views we couldn't achieve with other methods. For us, their technology was invaluable, not only for visualizing intricate structures but also for quantifying and analyzing cells in both normal and disease states."
TD: Collaborating with LifeCanvas has been incredible. Within just a month of sending them our tissue samples, we received stunning, detailed videos and stills of the spinal cord and heart. The quality and clarity of these visuals have been transformative for our research, providing views we couldn’t achieve with other methods. For us, their technology was invaluable not only for visualizing intricate structures but also for quantifying and analyzing cells in both normal and disease states.
LC: How do you see light sheet imaging benefiting your future research?
TD: Light sheet imaging will be crucial for our next steps. So far, we’ve mainly done qualitative analysis, but we’re eager to dive deeper. Our new tools label specific subtypes of spinal motor neurons, which could help us understand the differences among the many types in the spinal cord—up to 30 in mice. Some are more vulnerable to diseases like ALS, and light sheet imaging can help us study their morphology and connectivity in detail.
With this technology, we can better analyze why certain neuron types are more susceptible to disease, potentially leading to new insights and therapeutic approaches. Combining light sheet imaging with our viral tools will also be beneficial for other researchers focusing on different circuits and cell types in neuroscience.
LC: What are you most excited about moving forward?
TD: I’m incredibly excited about advancing our understanding of human diseases and the brain. While rodent models remain important, the future of neuroscience and therapeutic development lies in studying the human brain directly.
A major challenge is delivering therapeutic tools to the human brain, as no current viral capsid can efficiently cross the blood-brain barrier via IV in humans. This is a significant obstacle for gene therapy. However, we can make immediate progress in transducing the spinal cord, which is less challenging. This opens up exciting possibilities for treating spinal cord-related conditions.