Dr. Erin Hisey is a postdoctoral research fellow in the Ressler lab at the McLean Hospital and Harvard Medical School.

LifeCanvas (LC): How did you arrive at your current research interests?
Erin Hisey (EH): I’m working on modeling early life trauma in mice and studying its effects on the brain. Specifically, I look at changes in the neurocircuitry of male mice that experienced chronic social defeat as juveniles.
Before joining the Ressler lab, I worked on the neurocircuitry of songbirds, and how juveniles develop their songs over time. While this aspect of bird behavior can be beautifully quantifiable, there aren’t many genetic tools, nor are there lines of birds allowing you to target specific cell types. These tools are much more robust for mice, which have been better characterized as models for early life trauma.
My initial research shows that “defeated” juvenile mice remember their experiences well enough to exhibit a strong behavioral phenotype in adulthood. During my efforts to replicate this result, I also found that these mice generalize fear: they react negatively not just to a male mouse that looks like their aggressor, but to any white mouse, regardless of sex and other physical characteristics. In the Ressler lab, there’s a wealth of knowledge on the circuits and cell types involved in different phases of fear conditioning – I would like to tie together new data on social aversive experiences and fear generalization back into these more familiar paradigms.
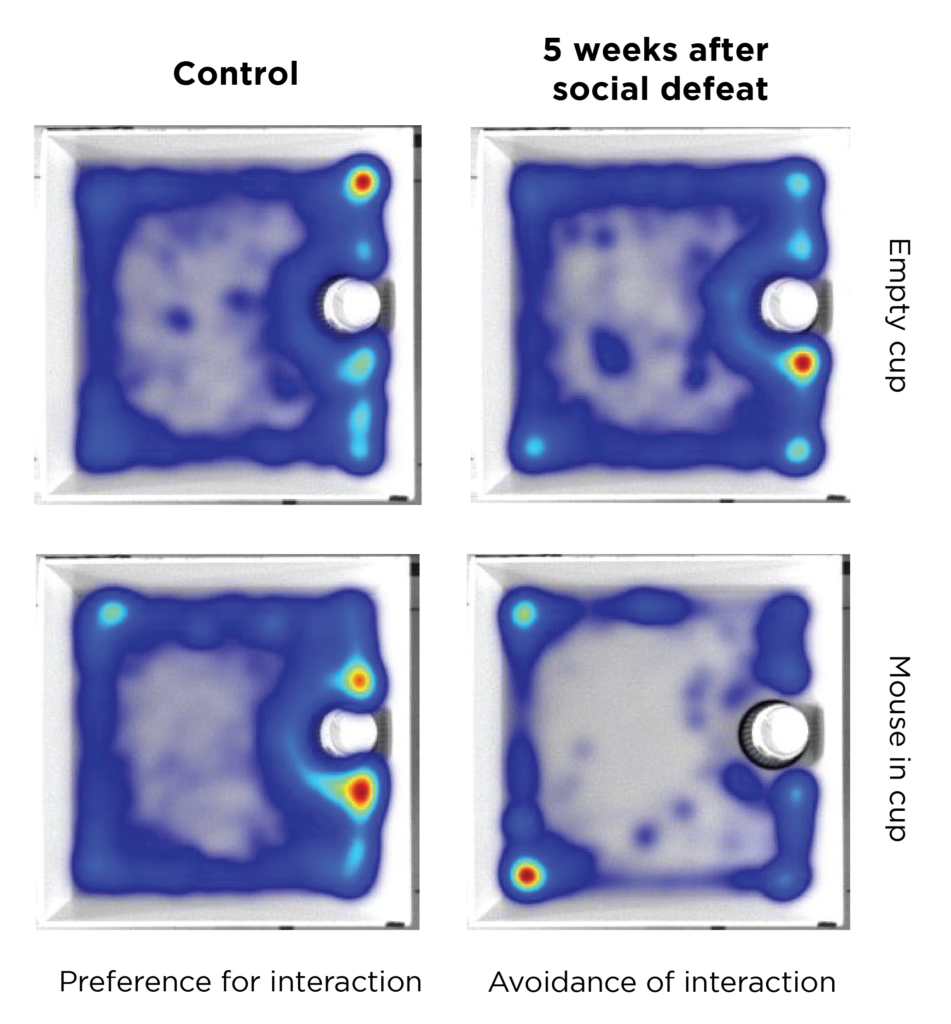
Compared to controls, mice that experienced early life “social defeat” showed aversion to other mice in their enclosure.
LC: What other notable effects of early life trauma have you seen in mice?
EH: In adult mouse models of social defeat, we find that some are naturally resilient: about half of them still want to interact with other mice after the defeat. However, when I model this in juveniles, only about ⅕ of the affected mice want to interact with others. Early life trauma seems to have a much stronger impact on social avoidance in adult mice.
Juvenile male mouse (black) and ovarectomized female mouse (white)
Before social defeat by white mouse, black mouse exhibits approach behavior including nasogenital contact
1 week after social defeat by white mouse, black mouse avoids white mouse and freezes or adopts upright defensive posture upon approach
Now we also have this touch screen task, kind of like a slot machine video game for mice, that allows us to study reward sensitivity in these models. The general idea is that one of the slot machines rewards the user 80% of the time, and the other rewards the user only 20% of the time. In control populations of both humans and mice, they prefer to use the 80% machine and ignore or interact less with the 20% machine. However in both species, individuals that were traumatized as juveniles don’t show this preference. They don’t develop this reward bias towards playing the game they’re more likely to win. In mice, we have also noticed that the “defeated” mice learn the task much more slowly than control mice.
LC: How has whole brain mapping with LifeCanvas’ services benefited your research?
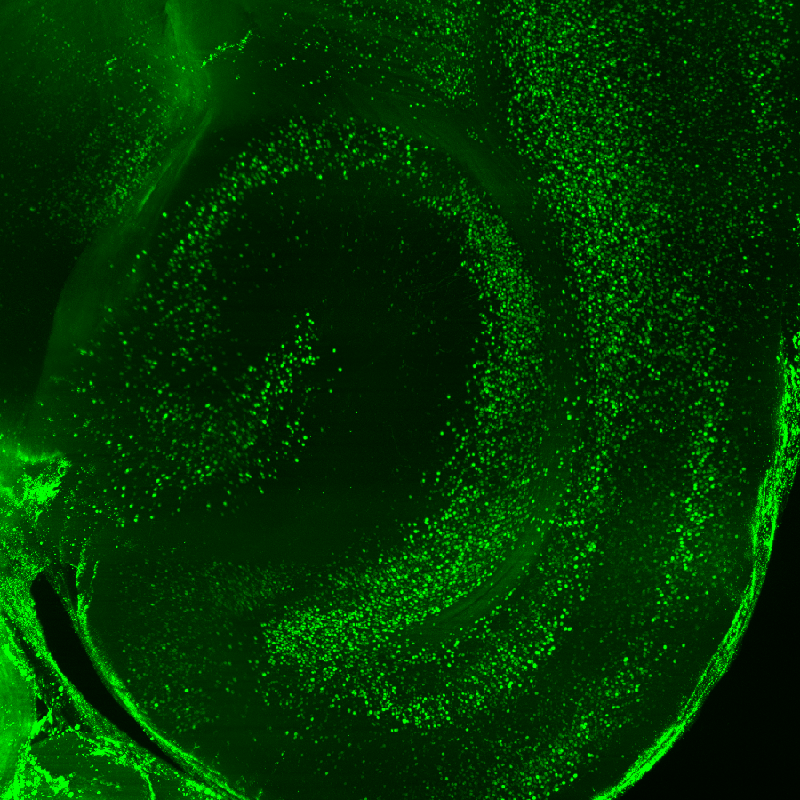
EH: With LifeCanvas’ help, I’m able to pinpoint brain areas potentially involved in social avoidance and try to dissect these implicated circuits. The whole brain approach provides a non-biased jumping off point for further investigation, without any a priori hypotheses. It would also be unfeasible to do it all by myself – I would have to restrain myself to just one small area. Once I’ve identified interesting regions across mice, I can tease apart the different circuits involved in the behavior of interest using optogenetics and likely also chemogenetics.
LC: What are your future research plans?
EH: I’d like to combine my knowledge of circuitry from imaging single cells during behavior with optogenetics or chemogenetics to manipulate relevant cell populations and see if they have a functional role in the behavior.
"The whole brain approach provides a non-biased jumping off point for further investigation, without any a priori hypotheses."
Since we’ve seen that CA3 has this striking upregulation in adult mice with early life trauma, we are also pursuing RNA sequencing to investigate the molecular identity of the cells that respond to the aversive social encounter. I hope this will reveal many differentially enriched targets, so that I can use CRISPR gene editing to knock down or reduce expression of these targets. I’m also interested in the cell types involved; c-FOS labeling and imaging can help identify candidate ensembles of CA3 cells for future experiments targeted at modulating generalized fear responses.
Eventually, I would be interested in studying this behavior in juvenile female mice with early life trauma. My colleague Emily has done amazing work studying aggression in females, and I’ve used modified versions of her methods to look at social defeat in juvenile females. LifeCanvas whole brain mapping would allow me to holistically compare sex differences in how these young mice respond.
LC: What are the translational implications of your work?
EH: At McLean we have the capacity to collaborate with labs doing clinical research, and in Kerry’s lab specifically we also have colleagues working with human patients. This allows us to compare activity in the brain regions we study in mice to the same regions in PTSD patients. Along with mouse samples, we also had the opportunity to send post-mortem samples from people with PTSD and early life trauma for RNA sequencing. Ideally, this will allow us to compare up- or down-regulation of gene expression across species. Similarly regulated targets could be good candidates for preclinical experiments aimed at reducing social fear in traumatized mice. There could already be drugs that target these sites. It’s exciting to think of the potential impact on human health.
