Dr. Emily Newman is a postdoctoral research fellow in the Ressler lab at the McLean Hospital and Harvard Medical School.
LifeCanvas (LC): What is your current research focus?
Emily Newman (EN): I’m interested in the cycle of violence as it relates to stress. In the past, I examined exposure to violence as a stressor which yields a maladaptive, perpetual phenotype that is often referred to as a “defeat” or “susceptible” phenotype. Now, I’m more interested in the aggressor side – I would like to understand the neurocircuitry that underlies this intersection between threat and aggression. More specifically, I’m interested in females as aggressors.

We’ve only recently figured out that female mice can become aggressive without maternal instinct as the driver. As we have only studied males in this context, this presents a unique type of aggression that may use different neurocircuitry.
LC: What interests you specifically about these sex differences?
EN: Creating a chronic social defeat stress model using female mice as both the aggressors and the animals that are defeated has the potential to be highly translational. Significantly more women than men are diagnosed with major depressive disorder, PTSD, and related disorders, in a way that is consistent across cultures and time, which suggests a possible biological component.
I’m also fascinated by the transition states within behaviors; pathology usually arises as the aberrant transition from one behavior to another. What is “normal” aggression in females would be deemed pathological in males, so the circuitries underpinning these phenotypes could be vastly different.
Non-aggressive and aggressive female behavior (white mouse): non-aggressive female exhibits approach behavior including nasogenital contact; aggressive female exhibits biting.
Another particularly interesting feature of the female aggression model is that these outbred mice naturally segregate into two separate populations — aggressors and non-aggressors — before any behavioral manipulation.
LC: How have LifeCanvas’ whole brain mapping services helped you achieve your research aims?
EN: We can deduce which brain areas might be sensitive to generating aggressor responses from electrophysiology studies, which involve inserting probes into specific brain areas. However, the full brain c-FOS mapping approach gives us an unbiased view of regions that we often overlook. This is especially instrumental in studying females, as we can’t just stamp our understanding of the male aggression phenotype onto these female mice. LifeCanvas’ approach gives us insight – and sight – into brain areas that could be good candidates for part of this female-specific aggression neurocircuitry.
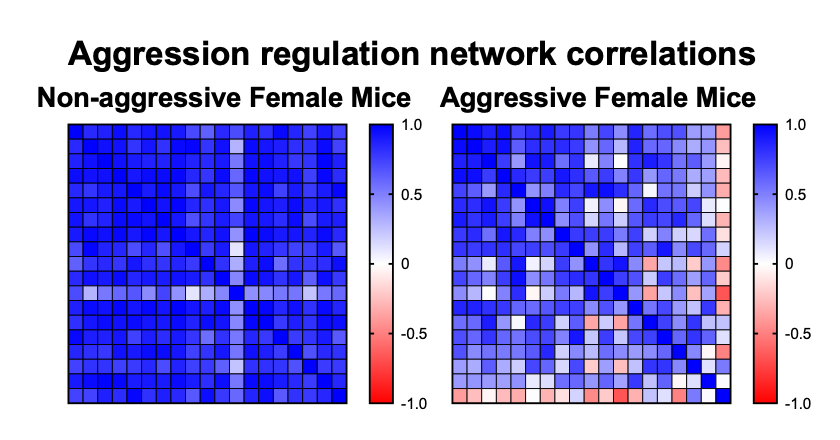
Aggressive female mice show whole-brain down-regulation of limbic regions.
Using this data and network analysis approaches, we have seen massive differences in these naturally segregating populations of aggressors and non-aggressors. Based on correlations between these populations, there seems to be whole-brain down-regulation of limbic regions in aggressors, compared to the relatively intact regulation network in non-aggressors.
LC: What are the translational implications of this work?
EN: Unfortunately, uncontrollable aggressive behavior is a common reason for emergency room admissions – more than half of emergency room nurses report being assaulted on the job. The main treatment to ensure patient and staff safety is currently sedation, which isn’t a long-term solution for most individuals. Sedative compounds make it difficult to function and disrupt the rest of your behavioral repertoire. I would ideally like to isolate a receptor population that might give us selective pharmacological tools to specifically treat aggressive behavior acutely and long-term.
"Applying a cross-species lens to collaborative studies will help us reveal conserved fundamental mechanisms that drive pathological aggression."
Using in vivo calcium imaging in mice, I’ve observed a population of cells within the central amygdala that seems to be controlling some aspects of a transition state between social investigation and aggressive behavior.
Dr. Jennifer Fanning, a clinical psychologist at McLean, has done compelling research on intermittent explosive disorder in women who have experienced violence. Considering the role of the central amygdala in both threat and aggression, I would be fascinated to see how her studies in humans could inform mine in mice. In addition, as a new member of the McLean Hospital research community, Dr. Caroline Palavicino-Maggio brings her perspective from female fruit fly aggression studies. Applying a cross-species lens to collaborative studies will help us reveal conserved fundamental mechanisms that drive pathological aggression.
LC: What are your future plans with whole brain mapping tools?
EN: I’m hoping to use whole brain mapping as an instruction manual for which downstream regions I could investigate with a closed-loop approach. This entails using calcium imaging combined with optogenetic stimulation to detect cell activity associated with a high probability of transitioning from non-aggressive social investigation into an attack; then, I can use that cell signature as a trigger to stimulate or inhibit those cells. This allows me to essentially stop the aggression as its precursor behavior is detected. Eventually, I would love to be able to see these whole networks in action in live behaving mice.

