As explained in several of our blogs, delipidation is a critical step in the tissue clearing process. Some form of lipid removal is present in almost all of the most popular clearing techniques, including CLARITY, iDISCO, SHIELD, and CUBIC. In the case of SHIELD and our technologies, the detergent Sodium Dodecyl Sulfate (SDS) is used to dissolve the lipids in the cell membranes to improve tissue transparency. This same mechanism is what makes soaps and detergents effective in destroying bacteria and viruses like coronavirus.
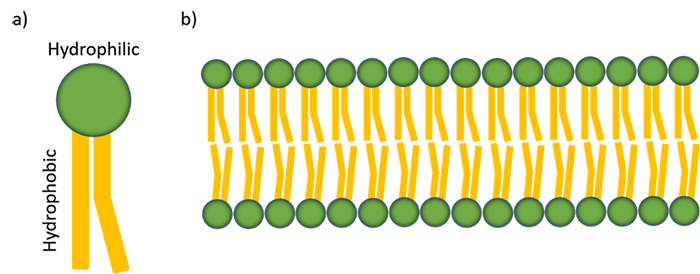
To better understand this mechanism, we should first examine the physical structure of the lipid membrane and the detergent itself. The lipid membrane present in mammalian cells is known as a phospholipid bilayer. This bilayer is composed of individual phospholipid monomers with a wide variety of proteins and other molecules scattered on and through it (Figure 1). These phospholipid monomers have a hydrophilic head and a hydrophobic tail. When the bilayer assembles, it is arranged such that the hydrophilic heads face the aqueous environments of the cytoplasm and extracellular space, while the hydrophobic tails are protected from water in the center of the bilayer.
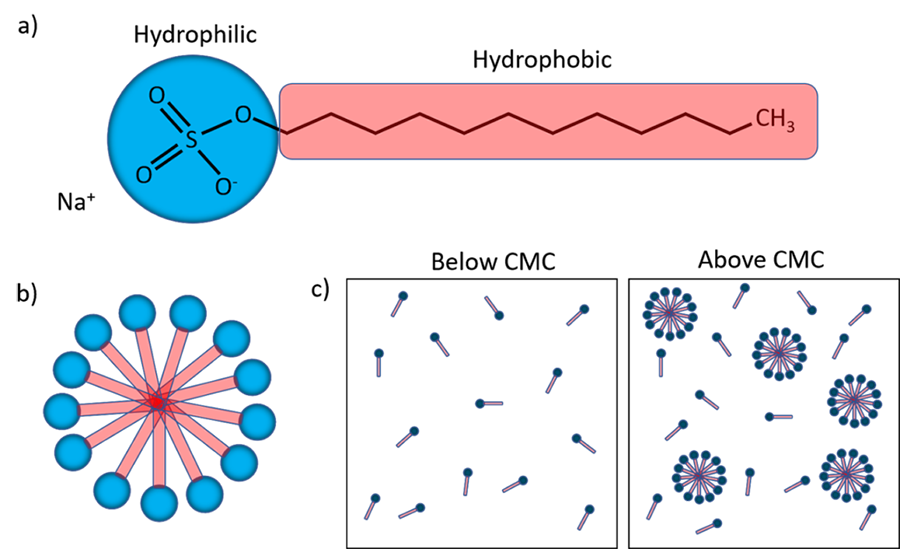
In aqueous solutions, detergents exist in two main forms: monomers and micelles. When the concentration of the detergent in water is low, all of the detergent is in its monomeric form. These monomers look quite similar to phospholipids as they also have hydrophilic heads and hydrophobic tails (Figure 2a). As the concentration of detergent increases the solution will eventually reach a point called the critical micelle concentration, or CMC. At this point, larger clusters of detergent begin to spontaneously assemble into micelles (Figure 2b). The CMC is different for every detergent, but for SDS it is ~ 8 mM [1]. Some detergent is still present in the solution as monomers, but any additional detergent that is added will form additional micelles (Figure 2c). The micelles form following similar principles to that of the phospholipid bilayer, and the hydrophilic heads assemble to protect the hydrophobic tails from the water in the environment.
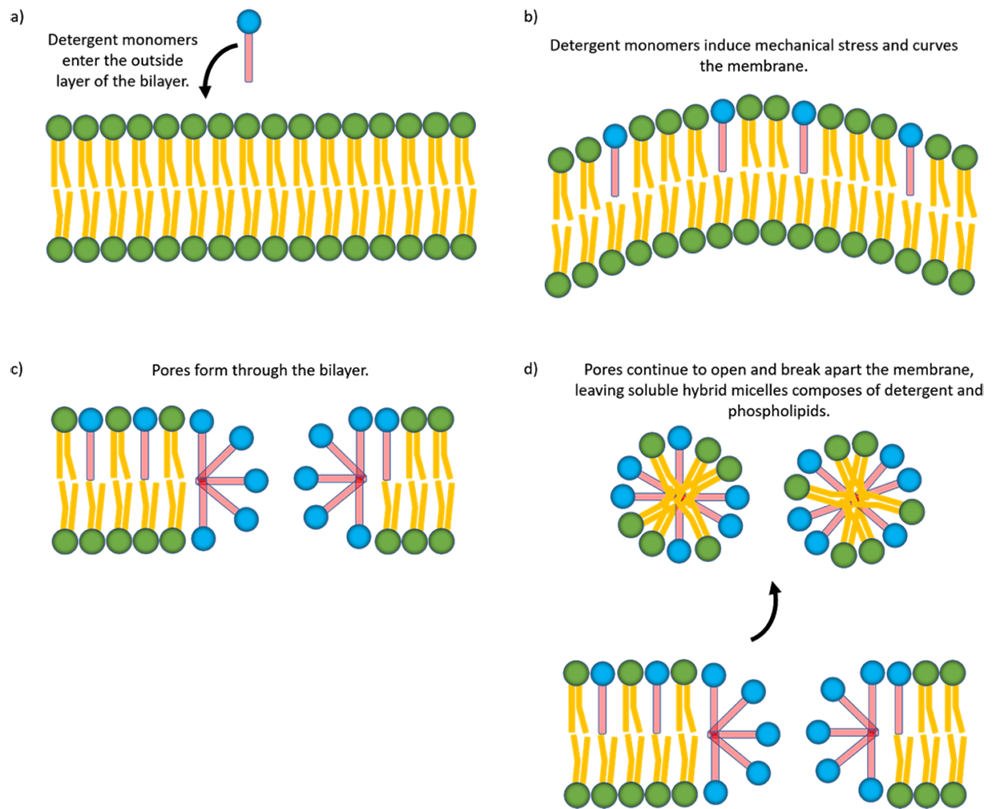
So how does the detergent actually dissolve the lipid membrane? This is a topic of significant research, and the most common mechanism follows a 3 step process [2]. The exact mechanism depends on the properties of the detergent, but for the purposes of this article, we will focus on SDS. First, detergent monomers enter the outside layer of the phospholipid bilayer, arranged similarly to the phospholipids according to the hydrophobicity (Figure 3a). The inserted detergent monomers induce a mechanical strain on the layer, and can physically bend it (Figure 3b). This happens with SDS because it has a slow flip-flop rate. This means that it takes a long time (minutes-hours) for monomers to flip from the outside to the inside of the bilayer. So, they build up on the external side of the membrane. Next, the bilayer reaches a breaking point due to the strain, and this forms a pore in the membrane (Figure 3c). Finally, monomers continue to distribute among the porous structure, and form hybrid micelles of phospholipid and detergent. This completely breaks apart and dissolves the membrane (Figure 3d).
This process takes time, as the reactions are not instantaneous, and it requires diffusion of monomers and micelles into the sample. For example, it can take about a month to fully delipidate a whole mouse brain with SDS. Luckily, this process can be sped up tremendously using electrophoresis, as with SmartBatch+. In water, SDS dissolves into positively charged sodium ions and the negatively charged detergent monomers and micelles. Under the presence of an electric field, these charged molecules experience an electrostatic force which provides additional acceleration into the tissue. This means the process is no longer relying on the passive diffusion of molecules into the tissue. This can provide a speed increase of roughly an order of magnitude and increase your scientific throughput. With the device, you will no longer need to wait about a month for the samples to clear to check the results of your experiments, thus allowing you to make observations more efficiently.
References
- P. Mukerjee, P. & Mysels, K. J. (1971), “Critical Micelle Concentration of Aqueous Surfactant Systems,” NSRDS-NBS 36, Washington, DC: US. Government Printing Office.
- Sudbrack, T. P., Archilha, N. L., Itri, R., & Riske, K. A. (2011). Observing the Solubilization of Lipid Bilayers by Detergents with Optical Microscopy of GUVs. The Journal of Physical Chemistry B, 115(2), 269–277. doi: 10.1021/jp108653e