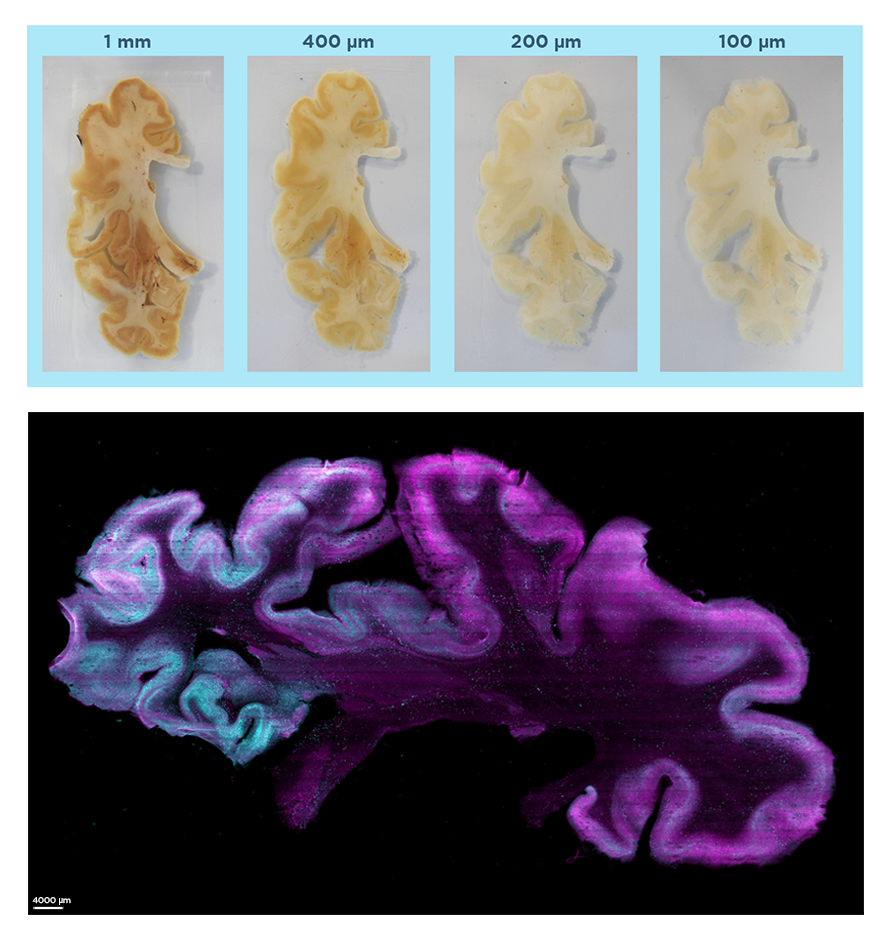
Megatome is a novel microtome that allows for high-precision sectioning of a wide range of tissue samples – from organoids, to arrays of animal organs, to intact human brain hemispheres – with minimal tissue damage and information loss. By combining high-frequency, high-amplitude blade vibration with low out-of-plane blade deflection, Megatome generates uniform, accurate sections of soft-gel embedded samples.
Megatome is the only instrument of its kind to enable high-precision, damage-free sectioning of human- and nonhuman primate-scale samples, offering researchers unprecedented opportunities to closely study these tissue types at scale.
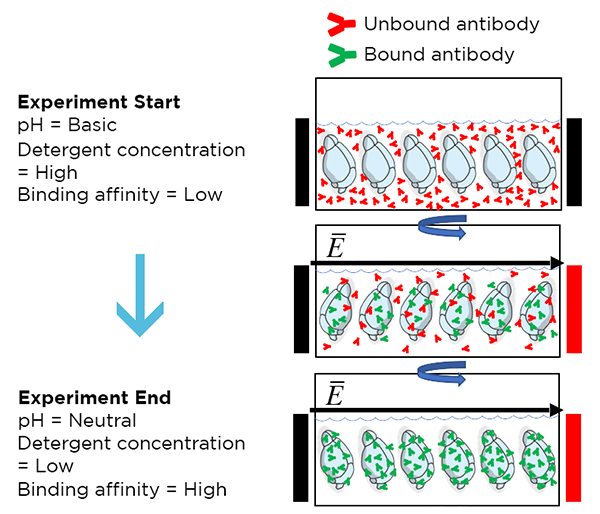
eFLASH is a rapid tissue labeling technique that allows for uniform whole-organ staining in <24 hours. This method combines stochastic electrotransport with modulation of antibody binding affinity via reaction conditions to enable uniform antibody diffusion without saturation at surface targets.
Initially, high pH and high NADC concentration prohibit antibody binding. During the experiment, pH and NADC concentration are gradually lowered and antibody affinity gradually increases, leading to antibody binding as antibodies are driven in by the electric field. By the end of the experiment, pH has been neutralized, NADC concentration is low, and most antibodies are uniformly bound throughout the sample.
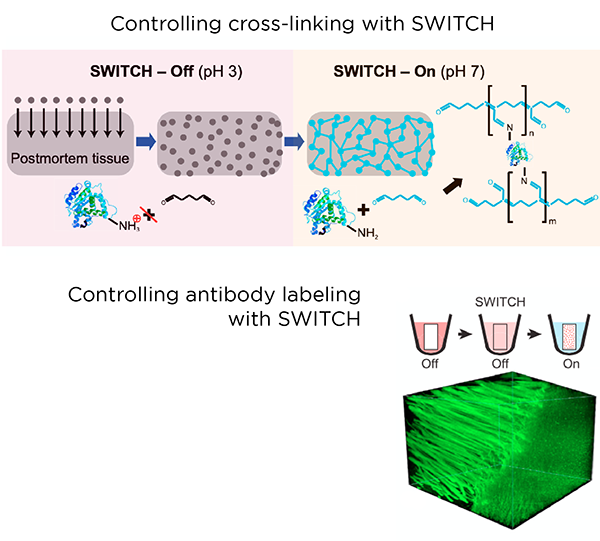
SHIELD is a tissue preservation technique that complements and enhances the standard PFA fixation of biological tissue. It requires a series of incubation steps over 4-6 days to preserve a tissue sample the size of a whole mouse brain. These steps allow epoxide molecules to diffuse into the tissue and crosslink, forming a strong skeleton that anchors proteins, enzymes, nucleic acids, and other important elements of tissue architecture.
This preparation step is necessary for active clearing or immunolabeling, as exposure to harsher chemicals or environments can strip away important targets in non-SHIELD-preserved tissue. The strong fixation of native proteins also allows for multiplex labeling.
The SWITCH method introduces a simple process for controlling a wide variety of chemical reactions during tissue processing of large animal and human samples. SWITCH ensures uniformity of preservation and immunolabeling via synchronization of chemical reactions throughout the entire sample.
There are 2 basic steps:
Tissue architecture, native biomolecules, and antigenicity are highly preserved, allowing for >20 rounds of labeling.
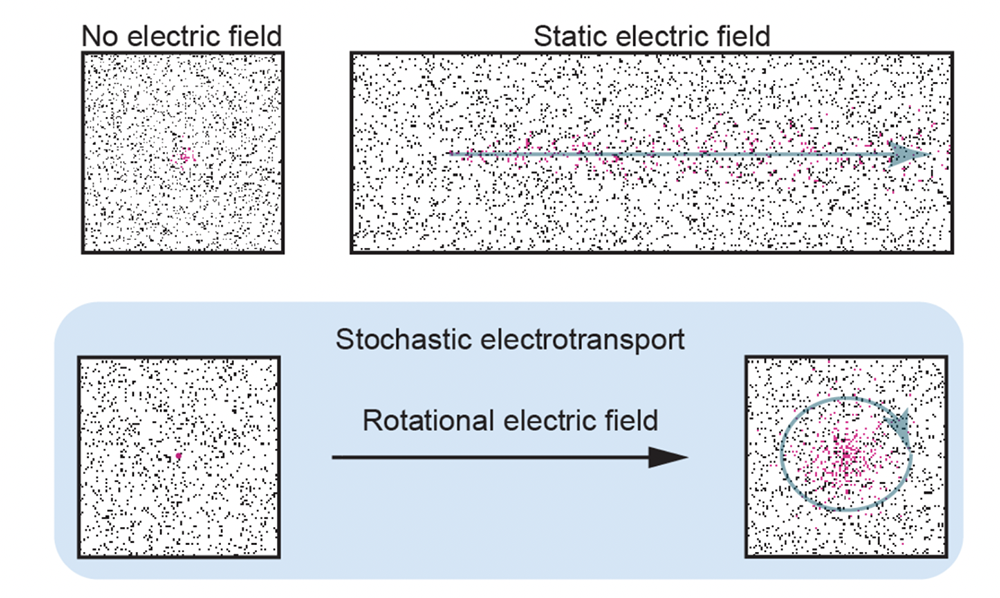
Stochastic electrotransport (SE) is a patented, novel method for rapid, nondestructive processing of porous samples. Processes like delipidation and immunolabeling have historically been limited by diffusion time, particularly for large samples such as intact mouse brains. Antibodies may take weeks to diffuse through only a few millimeters of tissue, with a steep labeling gradient from surface to core.
SE uses a rotational electric field to disperse highly electromobile molecules (such as antibodies or surfactant micelles) throughout a porous sample without damaging electrically charged structures within the tissue. This enables 2-4 day clearing of intact organs, <24 hour staining with nuclear dyes, proteins, and antibodies, and incredible labeling uniformity.
For technical support reach out to our dedicated customer support team at support@lifecanvastech.com.