Delipidation Appendices
Liver Clearing Protocol
In our hands, liver samples are somewhat cloudy after index matching when using our traditional delipidation protocol. To improve clearing, we add an additional round of SDS clearing following the Clear+ Delipidation Buffer incubation. This significantly improves clearing without causing damage to the sample.
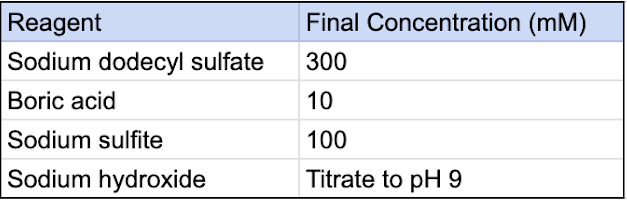
1. Prepare SDS clearing solution:
Reagents:
- Sodium dodecyl sulfate – Sigma-Aldrich 75746
- Boric acid – Alfa Aesar 12680
- Sodium sulfite – Sigma-Aldrich S0505
- Sodium hydroxide – Sigma-Aldrich S5991
2. For a whole mouse liver, incubate the tissue in SDS clearing solution for 2 days at 45°C. Smaller tissues or chunks can be incubated for shorter times, while larger tissues will require longer incubations.
3. Move the sample to PBSN (PBS with 0.02% sodium azide) and wash for 2 days at RT before continuing to immunolabeling, or before storing samples at 4°C. If the sample is not washed before going into 4°C, SDS can precipitate inside the tissue.
fDisco Processing
For samples containing connective tissue, adipose tissue, or skin tissue, an additional fDISCO step improves clearing of these other tissue types without damaging fluorescent proteins.
You will need the following solutions for this protocol:
- Dichloromethane (270997, Sigma Aldrich)
- Tetrahydrofuran (186562, Sigma Aldrich)
- Quadrol (122262, Sigma Aldrich)
Please note that dichloromethane and tetrahydrofuran are volatile organic solvents and should be handled with appropriate PPE in a fume hood. Samples should also be processed in polypropylene tubes as these chemicals can dissolve many plastics.
1. Prepare a solution that is 25% Quadrol, 75% PBS. We will call this solution PBSQ. Note that quadrol is very viscous. Once this solution is made, store it at 4°C.
2. Dehydrate the sample using a stepwise incubation of tetrahydrofuran (THF) and PBSQ. This should be done in 5 mL tubes filled all the way up. Incubate the sample as follows:
- 50% THF, 50% PBSQ, 1 hour at 4°C.
- 70% THF, 30% PBSQ, 1 hour at 4°C.
- 80% THF, 20% PBSQ, 1 hour at 4°C.
- 95% THF, 5% PBSQ, 1 hour at 4°C.
- 95% THF, 5% PBSQ, 1 hour at 4°C.
- Dichloromethane, 30 minutes at 4°C.
- Refresh dichloromethane and incubate further at 4°C. For whole mouse heads and similar sized samples, incubate overnight, then refresh the solution again, and incubate for 24 more hours. Smaller samples may only need overnight.
3. Once the sample has been dehydrated and treated with dichloromethane, it needs to be rehydrated. Incubate the samples in 5 mL tubes filled all the way up as follows:
- 95% THF, 5% PBSQ, 1 hour at 4°C.
- 95% THF, 5% PBSQ, 1 hour at 4°C.
- 80% THF, 20% PBSQ, 1 hour at 4°C.
- 70% THF, 30% PBSQ, 1 hour at 4°C.
- 50% THF, 50% PBSQ, 1 hour at 4°C.
4. Move the sample to PBSN, and wash for at least 24 hours at RT, with several PBSN refreshes before continuing with labeling steps.
